THE NEW MACDONALD PHARM
(August 2003)
Imagine a typical drive through the countryside. As you look out your window, you notice a farm. There are cows and sheep grazing on grass, chickens in the coop and pigs in the sty. However, this is no ordinary farm. What you don’t see is a blood clotting factor being produced in the cow’s milk, strong yet light-weight spider silk proteins being produced in the sheep, vaccines in the chicken eggs and that the pigs are destined to be a source of transplant organs (xenotransplantation). No, this is no ordinary farm. This farm of the future is actually a “pharm.”
Pharming, yet another cleverly misspelled word produced by the biotechnology sector, refers to using transgenic animals (animals that carry foreign genes in their genome) to produce drugs or other products beneficial to humans [1]. While this idea may not be that new, its implementation is just now beginning to take shape. Numerous companies are springing up all over the world marketing a plethora of these pharmaceutical products. With our current knowledge of what affects embryonic development being limited, the major hurdle to the success of these products is the ethical issues that have arisen through the use of transgenic animals, especially if they are to be used for human consumption.
A Word About Cloning
Cloning. Mention the word ‘cloning’ to the average person and often they will tell you the harms of cloning-for example, how it can be used to generate a population of genetically superior “super-humans.” However, often the most talked about applications of cloning are those that, in reality, are still fictitious. In reality, even current cloning techniques to creating transgenic animals are very difficult [2]. First and foremost, producing transgenic animals is very costly, ranging in price tags from $20,000 to $300,000. Furthermore, not all transgenic animals produce proteins in large amounts, or they produce them in the wrong tissues. So, if a transgenic animal were produced that expressed the correct protein, in the right tissue, and in large amounts, it would be beneficial if its offspring were to do the same.
How to Make Your Own Transgenic Animal
While the technique of cloning has changed dramatically over the years, the basic principle remains the same [2]. Take a blood clotting factor protein we would like to clone in a cow as an example. Essentially, the gene for the factor would be linked to a promoter specific for a milk protein. This promoter is responsible for ensuring that the gene is only expressed in the milk of the animal. Many copies of this promoter-gene combo are then introduced into a cow’s egg, which is allowed to develop in a surrogate animal (see Figure 1). A technique called ‘Pronuclear injection’ is used to do this and involves injecting the DNA directly into fertilized eggs using a very fine glass needle ( transfection).
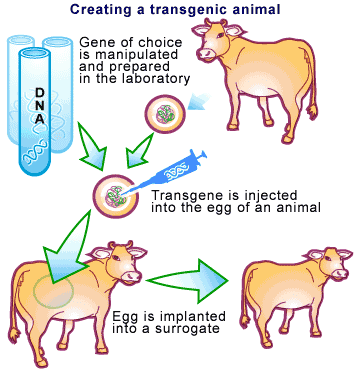
However, pronuclear injection is very unreliable. This is due to DNA uptake and integration into the genome of the egg cell being a very rare event, typically resulting in 1 to 5 transgenic animals out of every 100 being born. Dolly the sheep, the first animal cloned by pronuclear injection, was the result of one success out of 277 eggs [3]. Another Biotech company, PPL Therapeutics, took several years just to produce a flock of 600 transgenic sheep.
It was obvious that if pronuclear injection were the only animal cloning technique available, pharming would not be a feasible option. This problem has been solved by a new technique called ‘nuclear transfer’, which solves the low percentage of transgenic offspring by selecting for eggs that have cloned genes integrated into the egg’s genome before it is implanted into the surrogate [4] (see Figure 2) Essentially, cells are transfected as before, with the addition of a gene that makes the egg resistant to an antibiotic if it is expressed properly. This allows for the selection of only those cells that properly express the antibiotic resistance gene, and in conjunction, the gene of interest. The nucleus of these cells are then removed and transferred to an egg that has had its nucleus removed, followed by treating and culturing the egg to allow fusion with the nucleus. Since all the eggs contain the transgene, virtually 100% of the offspring will be transgenic animals.
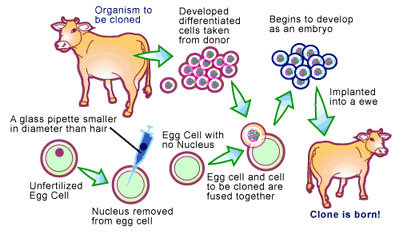
Problems with Modern Cloning Techniques
While nuclear transfer practically guarantees a litter of transgenic offspring, problems still exist with this method of cloning. First of all, not only is integration of the transgene into the genome a very rare event, it is also random, as scientists have no way of directing where the transgenes end up [4]. This means that the transgene could insert into regions that may be deleterious to its expression or it may even insert into an endogenous gene (a gene belonging to the animal) knocking out its function. Secondly, many of the animals are born with gross birth defects. These animals can be grossly over or under weight, and many have internal organs that are underdeveloped or deformed. Therefore, even though scientists can produce a full litter of transgenic offspring, there is no guarantee that these animals will be (a) healthy, (b) express the transgene in large amounts, or (c) express the transgene in the right tissue.
While scientists have greatly improved the techniques used to produce transgenic animals, cloning is still essentially a trial-and-error procedure. These problems doomed one biotech company, Granada Inc., which tried to make a herd of transgenic cattle. They were forced to shut down, as they could not generate enough calves [3]. Scientists are still baffled by the high rate of birth defects, but they point out that animals born by in vitro fertilization also tend to be born with a high rate of birth defects (albeit at a lower frequency) [3]. This suggests that it is not necessarily the nuclear transfer that is causing the defects, rather how the implanted embryo develops in the surrogate womb. Thus, the search for the ability to target integration of the transgene, and improved methods of culturing and implanting the embryo continues.
Why Pharm?
Outlined above are ‘the what’ and ‘the how’ of pharming, plus some of the physical problems associated with it. Despite the expense and difficulty in creating a successful transgenic animal, the benefits can still potentially outweigh the setbacks. For the biotech company that is producing transgenic animals, and for its investors, the benefits are obvious — money, and lots of it from pharmaceutical sales. However, pharming can benefit the ordinary person by reducing the cost of pharmaceuticals. Currently, the method of choice for manufacturing many drugs is by laboratory cell culture of bacteria, yeast, or animal cells [5]. There are many drawbacks to this way of mass producing drugs: (a) cell cultures require constant monitoring and sampling, as cell cultures tend to require precise parameters in order to produce large amounts of protein, (b) expansion is very costly and laborious, as new equipment is required, as is space to store the equipment, (c) in order to retain biological activity, many proteins require modifications (addition of sugars, for example), some of which are only performed by mammalian cells, and (d) most cells need to be ruptured (not a trivial procedure if you consider the size of modern bioreactors) to isolate and purify the protein of interest, which is much more difficult than purifying proteins from the blood or milk of an animal. So, even though the initial cost of producing transgenic animals is quite high, using animals as bioreactors is actually a cost efficient alternative to mass produce human pharmaceuticals. One estimate by AviGenics (a private biotech firm, so take these numbers with a grain of salt) states that while modern cell culture costs roughly $100 per gram of protein produced, pharming the same protein cost roughly $2 to $20 per gram if using goat milk, or $0.10 to $0.25 per gram if using poultry eggs [2]. This will mean that the consumer will benefit from receiving pharmaceutical drugs at a fraction of the cost.
Some Pharming Projects of Interest
When the Roslin Institute and PPL Therapeutics announced the birth of Dolly in 1996, quite a stir was created. Dolly, a sheep, was the first animal cloned from an adult cell, previously believed to be impossible. A year later, the same group announced the birth of two more cloned sheep, Molly and Polly. However, these two sheep were very different from their cousin, Dolly. The most pronounced difference was the fact that they both carried the human gene that codes for a protein called Factor IX [3]. Factor IX is a blood clotting protein, and it is commonly used to treat patients that have hemophilia B, a disease that is caused by a deficiency in Factor IX. The goal of creating Molly and Polly was to produce a herd of sheep that produced Factor IX in their milk. The only other source of Factor IX is from human blood plasma. By producing Factor IX in sheep milk, it is hoped that the cost of production will be greatly reduced.
Advanced Cell Technology, Inc is using the queen of milk production, the cow, for potential use as bioreactors [4]. They have produced transgenic cows that secrete the protein serum albumin in their milk, a protein that is used to extend blood volume and is used in patients suffering from traumatic injuries, such as burns. Cows are an obvious choice for pharming purposes as they can produce upwards of 8000 L of milk per year, and an estimated 40 to 80 kg of protein a year [4]. That is quite a substantial amount compared to the 4 kg of protein per year in goats and 2.5 kg of protein per year in sheep [4].
One interesting project is that taken up by Nexia Biotechnologies. For years humans have wanted to employ the silk of spider webs for many applications, as the silk is strong, elastic, and extremely lightweight. However, unlike silk worms, spiders cannot be farmed for their silk, as spiders are extremely territorial, plus it is not the complete web that is desired, rather a portion of the web referred to as frame silk [4]. Cell cultures have been unsuccessful for producing spider silk, as they tend to produce fibres that are too short for commercial applications. Nexia Biotechnologies has been successful at producing spider silk in the milk of goats, and is in the process of developing “Biosteel®”, a man made fabric made of spider silk [6]. Potential applications of this fabric are extensive, ranging from medical uses (wound closures, dressing, patches, glues, prosthetic devices), to military uses (strong, light-weight body armour), to sporting goods (biodegradable fishing line).
Ethical Issues to Pharming
Many of the ethical issues that arise from pharming surround the treatment of animals [7]. Even with the 100% transgenic offspring produced by nuclear fusion, many are born with birth defects and gross abnormalities or do not produce the protein of interest. Additionally, while 100% of the animals born are transgenic, a large number of eggs are used in the process of finding one can be implanted. This in itself may not be too alarming, however, most of the time the egg “donor” is slaughtered in the donation process.
Another issue is the idea of the age of the clones [8]. Dolly recently passed away at the age of 6 and a half. Considering that the average sheep lives for 11 to 12 years, this was quite young. Dolly died from a lung disease found only in old sheep, adding to speculation that cloning animals may effect their age. In fact, many cloned animals tend to die young, some within weeks of birth. When the Roslin Institute and PPL Therapeutics announced the birth of Molly and Polly, they had a litter of six lambs out of 14 cloned embryos. One died within hours of birth, and three more died shortly after, leaving the world with Molly and Polly.
Finally, as transgenic animals are being produced, biotech companies are quick to patent their work in order to maximize their profits. This raises the issue of animal rights, and whether or not these animals will be treated as sentient beings or whether they will simply be treated as walking factories.
Conclusion
Pharming promises a world of cheap pharmaceutical drugs, new medical applications, and even the possibility of wearing your new spider silk jacket to the hippest bar in town. However many of the products can still be produced alternatively (i.e. cell culture) and while pharming promises to produce the same pharmaceuticals cheaper and better, that realism is too far away to determine whether or not it can be delivered. Moreover, the ethical issues surrounding the use of animals as pharmaceutical factories is still being debated, let alone new debates as new application of pharming are suggested. Pharming’s promise is exciting and the real farm is sure to new be the same.
Additional Reading
1. Clarke A.R., ed. (2002). Transgenesis Techniques: Principles and Protocols. Totowa, NJ: Humana Press. 351p.
2. Baldi P. (2001). The Shattered Self: The End of Natural Evolution. Cambridge, Mass: MIT Press. 245p.
3. Yount L. 2000. Biotechnology and Genetic Engineering. New York: Facts on File. 280p.
4. Rifkin J. 1998. The Biotech Century: Harnessing the Gene and Remaking the World. New York: Jeremy P. Tarcher/Putnam. 271p.
References
1. Rosenfeld A. (1998). New breeds down on the pharm: plain old barnyard animals — with genes from other species added — are producing medicines that keep people alive. Smithsonian 29(4): 22-30.
2. Rittner M., Cummings D. (1999). Animal Pharming: The Industrialization of Transgenic Animals. Centers for Epidemiology and Animal Health:
3. Pennisi E. (1998). After Dolly, a Pharming frenzy. Science 279: 646-648.
4. Dove A. (2000). Milking the genome for profit. Nature Biotechnology 18: 1045-1048.
5. Whitehouse D. Doubts over ‘pharming’ technology. BBC news. March 25 2002:
6. Nexia Biotechnologies. General Information about Spider Silk
7. Fox M.W. (1999). Beyond Evolution: Altered Future of Plants, Animals, the Earth, and Humans. New York, NY: Lyons Press. 256p.
8. Sgaramella V., Zinder N.D. (1998). Dolly Confirmation. Science 279: 635.
(Art by Jane Wang – note that high res versions of image files available here)