MUCOSAL IMMUNITY AND VACCINES
(August 2003)
The mucous membranes are one of the largest organs of the body (Figure 1). Collectively, they cover a surface area of more than 400m2 (equivalent to one and a half tennis courts) and comprise the linings of the gastrointestinal, urogenital and respiratory tracts [15,18].
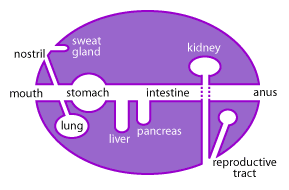
These mucosal surfaces, while located inside the body, are actually a physical barrier between the outside and the sterile interior cavity of the body known as the “systemic” environment. Critical nutrients, oxygen and other molecules are constantly taken up across these mucosal barriers; however, another important function of the mucous is to keep invading pathogens out [15]. Daily these mucous membranes are bombarded by outside elements and it is up to the unique immune system of the mucous to determine what is potentially harmful and what is beneficial [15,18].
Why mucus matters
Interest in mucosal tissues stems from their importance in immunity, with the vast majority of human pathogens initiating infections at mucosal surfaces, making the gastrointestinal, urogenital and respiratory tracts major routes of entry into the body [14,18]. In fact, virtually the only way to contract an infection other than mucosally is through blood-borne routes such as injections, transfusions and bites. Examples of mucosally-infecting agents include cold viruses, influenza, food poisoning agents, tuberculosis, sexually transmitted diseases, cholera, diphtheria and plague [15]. Despite its important role, only a handful of vaccines specifically target this area of the immune system despite strong evidence that a good mucosal response can effectively prevent systemic infections [15].
Components of a mucosal immune response
The systemic and mucosal immune systems have an arsenal of molecules specifically designed to eliminate invading pathogens. One of the first lines of defense are antibodies, made and secreted by plasma B cells. Antibodies are large protein molecules capable of binding and neutralizing an invading organism7. A picture of a typical antibody is shown in Figure 2.
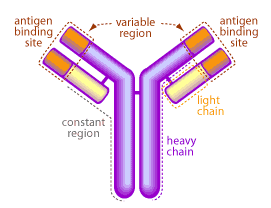
The mucous membranes produce a special type of antibody called secretory IgA or sIgA. In the mucous, this antibody is secreted as a dimer, joined at the non-antigen binding end by a protein known as the J chain as shown in Figure 3.
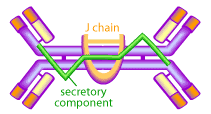
This form of the antibody is more stable, less resistant to proteolysis by the digestive enzymes of the gut, and has higher avidity for mucosal surfaces [13,15,18]. The mucous membranes are bathed in huge quantities of sIgA, which act as a first line of defense to neutralize invading pathogens [15,15]. Experimental evidence shows that the presence of sIgA correlates with resistance to infection by various pathogens, including bacteria, viruses, parasites and fungi [13,15,18]. It has also been shown to neutralize viruses and prevent their adherence to the epithelial cells lining the mucous (thereby preventing infection) as well as mediating excretion of pathogens and preventing the assembly of mature virus particles [13,15,18,21].
Another important component of mucosal immunity is the T cell-mediated immune response. T cells that specifically recognize pathogens can help antibodies to clear the infection or directly kill the invader themselves. T cells produced in the mucous are capable of traveling throughout the mucosal tissues through special “homing” receptors on their membranes [13,15,18]. This means that if an immune response is generated in the gastrointestinal lining, T cells produced there can travel to other mucosal sites, for example, the lungs or nasal cavity, providing protection over a large surface area [13,15,21].
The importance of mucosal immunology lies in the interplay between the mucosal response and the systemic immune response. Several studies have demonstrated that stimulating the immune system systemically (i.e. via injection or blood-borne routes) results in production of protective antibody and T cells only within the sterile, internal environment of the body—no mucosal response is generated. On the other hand, stimulation of the mucosal immune response can result in production of protective B and T cells in both mucosal and systemic environments so that infections are stopped before they get into the body [13,15,18].
Mucosal vaccinations
The goal of current mucosal vaccination strategies is to prevent both initial stages of disease (colonization and infection by pathogens) and block its development [14]. Currently, the vast majority of vaccines available only block disease development once the pathogen has crossed the mucosal barrier into the normally sterile systemic environment [13]. Mucosal vaccines have several advantages over traditional systemic vaccines. They can be administered orally or nasally rather than via injection. This is more widely accepted by the public, as well as making the vaccine simpler to administer and distribute. In addition, there is less risk of needle stick injury or cross-contamination [13,15,18]. Inoculation could be accomplished using recombinant bacteria to express pieces of proteins from the pathogen (known as antigens) that the immune system will respond to and “remember” for the next infection. Another possibility is the creation of transgenic plants expressing pathogen antigens that could be eaten to administer the vaccine. Certain types of transgenic plants such as banana trees and potatoes have also been modified to overexpress protein antigens from pathogens such as hepatitis B and rotavirus; however, to date the level of protein expressed in the plant is variable and often too low for effective immunization [13,14,15].
Spotlight on a mucosal AIDS vaccine
Studies of populations with high incidences of HIV infection have shed some light on possible approaches to the development of an effective vaccine. Analysis of several groups of HIV-exposed individuals has consistently revealed the existence of a small subgroup that is resistant to infection despite multiple, long-term exposures [11,12]. In about 30% of cases it appears to be the presence of high levels of HIV-neutralizing sIgA in the genital tract and HIV-reactive T cells in the cervix that confers resistance to infection [10-12]. Because 70-80% of people contract the disease mucosally, and because the gut is the major reservoir for HIV replication, there is interest in the potential of the mucosal immune response in preventing and/or clearing infection [2,10]. Among the newer vaccines designed to induce a protective immune response to HIV is one designed to mimic the mucosal responses seen in these resistant individuals [2]. In 2001, a US group tested the first mucosal HIV vaccine in rhesus macaques [2]. While the vaccine showed promising results, it failed to prevent infection.
Autoimmunity
Autoimmunity occurs when the immune system begins attacking “self” components of the body aberrantly. The reasons why this occurs remain largely unknown, but the consequences can be devastating as parts of the body are slowly destroyed by the very system designed to protect them. Examples of autoimmune diseases include multiple sclerosis, rheumatoid arthritis, lupus and type I diabetes [15]. Mucosal immunity offers some possibilities for alleviating or reversing symptoms of autoimmune diseases. The basis of treatment involves a phenomenon known as mucosal tolerance. In the 1940’s, M.W. Chase noticed that repeated feeding of a substance to mice reduced their immune response when the same substance was injected systemically [15]. His experiment is outlined in Figure 4.
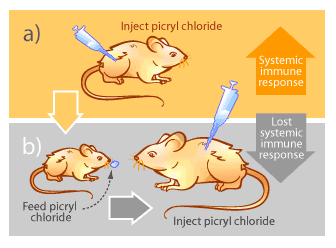
This phenomenon, known as mucosal tolerance, has been demonstrated not only with picryl chloride, but also with heavy metals, proteins, blood cells, mites, inactivated viruses, bacteria and many other substances [15]. The exact mechanisms of how the body adapts its immune response in this way are still not well understood [15]. Nevertheless it is believed to be a normal body function to prevent hypersensitivity to food proteins, normal bacterial flora that inhabit the gastrointestinal tracts and other mucosal surfaces of healthy individuals, and other environmental macromolecules [15].
Mucosal tolerance is being exploited for the development of autoimmune disease therapies. Because autoimmunity is caused by the immune system reacting to “self” antigens, the theory is that administration of the self antigen to which the body is aberrantly reacting by oral or nasal routes could induce mucosal tolerance and thus lower the harmful systemic immune response [15]. For example, in multiple sclerosis (MS), myelin (a fatty substance surrounding nerve cells) is slowly degraded by the immune system, resulting in gradual loss of nerve function (see Figure 5 and http://www.mssociety.ca/).
Mice suffering from EAE (acute experimental autoimmune encephalomegalitis) have very similar symptoms and are used as experimental models for human MS. Myelin deterioration was suppressed in these mice by oral or nasal administration of very high doses of MBP (myelin basic protein) [6,9,15]. Clinical trials in humans using bovine MBP have had controversial results: MBP reappeared on nerves of patients in a pilot study but with a larger group the positive observations of the initial study failed to be confirmed [6,9,15].
Immunocontraceptives
The idea of harnessing the immune response to control fertility was first proposed in the 1930’s [3]. However, only recently has our comprehension of the immune system progressed to the level where the idea may finally be put into practice. Over the past 30 years, the World Health Organization Task Force on Vaccines for Fertility Regulation has supported basic and clinical research on the development of birth control “vaccines” [8]. Studies have shown that approximately 30% of human infertility is associated with the presence of anti-sperm antibodies in mucous secretions of men and/or women and sperm-reactive T cells in men only [3,16]. These individuals show no other health problems other than immune-mediated infertility: therefore, inducing an immune response against critical components of reproduction might be a safe and effective way to control conception [3,16].
One approach being considered is the design of a “vaccine” capable of inducing an immune response against sperm antigens in either partner. Immunization of the male partner would result in inviable sperm production, while immunization of the female partner would result in reduced sperm motility or neutralization in the cervix and/or impaired penetration of sperm into the oocyte [3]. Another target for immunocontraceptive vaccines are reproductive hormones such as follicle stimulating hormone and human gonadotropin. Interfering with the functioning of these hormones can also reduce fertility.
References and Additional Reading
1) Barber B. Introduction: Emerging vaccine strategies. Sem. Immunol. 1997 9: 269-270.
2) Belyakov IM, Hel Z, Kelsall B, Kuznetsov VA, Ahlers JD, Nacsa J, Watkins DI, Allen TM, Sette A, Altman J, Woodward R, Markham PD, Clements JD, Franchini G, Strober W, Berzofsky JA. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat Med. 2001 Dec;7(12):1320-6.
3) Delves PJ, Lund T, Roitt IM. Antifertility vaccines. Trends Immunol. 2002 Apr;23(4):213-9. Review.
4) Delves PJ, Lund T, Roitt IM. Future prospects for vaccines to control fertility.Trends Immunol. 2002 Apr;23(4):220-1.
5) Fujihashi K, Koga T, van Ginkel FW, Hagiwara Y, McGhee JR. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants.Vaccine. 2002 Jun 7;20(19-20):2431-8. Review.
6) Fukaura H, Kent SC, Pietrusewicz MJ, Khoury SJ, Weiner HL, Hafler DA. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-beta1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest. 1996 Jul 1;98(1):70-7.
7) Goldsby RA, Kindt TJ and Osborne BA. “Kuby Immunology” 4th ed. W.H. Freeman & Co: New York. 2000.
8) Griffin PD. The WHO Task Force on Vaccines for Fertility Regulation. Its formation, objectives and research activities. Hum Reprod. 1991 Jan;6(1):166-72. Review.
9) Hafler DA, Kent SC, Pietrusewicz MJ, Khoury SJ, Weiner HL, Fukaura H. Oral administration of myelin induces antigen-specific TGF-beta 1 secreting T cells in patients with multiple sclerosis. Ann N Y Acad Sci. 1997 Dec 19;835:120-31.
10) Kaul R, Plummer FA, Kimani J, Dong T, Kiama P, Rostron T, Njagi E, MacDonald KS, Bwayo JJ, McMichael AJ, Rowland-Jones SL. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J Immunol. 2000 Feb 1;164(3):1602-11.
11) Kaul R, Trabattoni D, Bwayo JJ, Arienti D, Zagliani A, Mwangi FM, Kariuki C, Ngugi EN, MacDonald KS, Ball TB, Clerici M, Plummer FA.HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS. 1999 Jan 14;13(1):23-9.
12) Mazzoli S, Lopalco L, Salvi A, Trabattoni D, Lo Caputo S, Semplici F, Biasin M, Bl C, Cosma A, Pastori C, Meacci F, Mazzotta F, Villa ML, Siccardi AG, Clerici M.Human immunodeficiency virus (HIV)-specific IgA and HIV neutralizing activity in the serum of exposed seronegative partners of HIV-seropositive persons. J Infect Dis. 1999 Sep;180(3):871-5.
13) McCluskie MJ, Davis HL. Mucosal immunization with DNA vaccines. Microbes Infect. 1999 Jul;1(9):685-98. Review.
14) Medina E, Guzman CA. Modulation of immune responses following antigen administration by mucosal route. FEMS Immunol Med Microbiol. 2000 Apr;27(4):305-11. Review.
15) Ogra PL, Faden H, Welliver RC.Vaccination strategies for mucosal immune responses. Clin Microbiol Rev. 2001 Apr;14(2):430-45. Review.
16) Rajalakshmi M, Griffin PD. Male Contraception: present and future. New Age International (P) Ltd: New Delhi. 1999. pp.309-328
17) Raychaudhuri S, Rock KL. Fully mobilizing host defense: building better vaccines.Nat Biotechnol. 1998 Nov;16(11):1025-31. Review.
18) Rosenthal KL, Gallichan WS Challenges for vaccination against sexually-transmitted diseases: induction and long-term maintenance of mucosal immune responses in the female genital tract. Semin Immunol. 1997 Oct;9(5):303-14. Review.
19) Talwar GP. Vaccines and passive immunological approaches for the control of fertility and hormone-dependent cancers. Immunol Rev. 1999 Oct;171:173-92. Review.
20) United Nations AIDS Information.
21) van Ginkel FW, Nguyen HH, McGhee JR Vaccines for mucosal immunity to combat emerging infectious diseases. Emerg Infect Dis. 2000 Mar-Apr;6(2):123-32. Review.
(Art by Jane Wang – note that high res versions of these image files are available here)