IDENTIFYING DNA, RNA AND PROTEINS: THE BLOTS
(August 2003)
The separation of DNA/RNA fragments or a protein sample based on size can be useful in many applications, but visualizing samples on a gel does not give any information about the identity of the sample (i.e. which gene fragment, mRNA or protein you are looking at). So how can you pick out the band you’re interested in? E.M. Southern answered this question when he developed a method that was subsequently named after him, the Southern Blot.
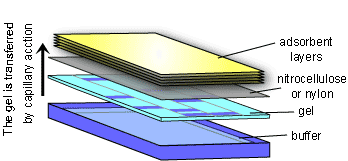
The Southern Blot takes advantage of the fact that DNA fragments will stick to a nylon or nitrocellulose membrane. The membrane is laid on top of the agarose gel and absorbent material (e.g. paper towels or a sponge) is placed on top. With time, the DNA fragments will travel from the gel to the membrane by capillary action as surrounding liquid is drawn up to the absorbent material on top. After the transfer of DNA fragments has occurred, the membrane is washed, then the DNA fragments are permanently fixed to the membrane by heating or exposing it to UV light. The membrane is now a mirror image of the agarose gel.
To figure out where the DNA fragment of interest is located, the membrane must be probed. A DNA fragment is synthesized that is complementary to the fragment of interest. The probe is also labeled (there are several ways of doing this: radioactively, with a fluorophore, biotin labeled, etc.) to allow identification. The membrane is washed with the probe, and if the probe finds its complementary strand of DNA on the membrane it will bind to it. The membrane is then washed to remove excess probe, and the membrane is viewed appropriately (i.e. if the probe was radioactively labeled, autoradiography is used to visualize the membrane). The location of the gene of interest can be determined easily.
The procedure for identifying RNA fragments is very similar, and in reference to E.M. Southern, this procedure was called the Northern Blot.
The method of identifying specific proteins is called the Western Blot and uses the same principles as the Southern Blot, but there are some important differences. First, the proteins are transferred to the nylon membrane by electrophoresis instead of capillary action. A current is applied across the gel/membrane complex to transfer the negatively charged proteins. Also, instead of a complementary oligonucleotide probe, Western blotting relies on a protein/antibody reaction. A primary antibody that is specific to the protein of interest is used as the probe. Then, a secondary antibody that is specific to the primary antibody is added. The label is attached to the secondary antibody. The primary antibody must be selected so that it is specific only to the protein you are interested in.
(Art by Fan Sozzi)