THE ROLE OF INTEGRINS IN WOUND HEALING
(August 2005)
Wound repair is a critical part of the maintenance of skin and mucosa. It involves a complex series of interactions and cooperation between the superficial skin cells (epidermal cells), cells from the lower layer of skin (dermal cells), and cells of our body’s defense system (immune cells) [1]. After the skin has been injured, the wound must be covered as rapidly as possible in order to prevent any infection. The first event upon wounding is bleeding, which shortly turns into clot. The process of wound coverage is called re-epithelialization, in which epidermal cells from the sides of the wound start migrating into the clot at the wound site. This migration starts as early as 24 hours after injury. Thereafter, those migrating epidermal cells start dividing and proliferating to complete the wound coverage. Integrins, a group of cell surface proteins, are important factors in this process. They facilitate the migration of epidermal cells into the wound area [2].
What are integrins?
Cell-surface receptors are proteins designed to transfer information from the outside of the cell to the inside [3]. One type of cell-surface protein receptor is the integrin, which is important in a variety of cellular functions such as growth, development, immune response, and wound repair. Integrins are the main way by which cells both bind to and respond to their environment [4]. Functional integrins consist of two glycoprotein subunits (protein structures that contain carbohydrate components), which are extended across the cell membrane connecting the inside of the cell to the outside. These subunits are called alpha chain and beta chain. Each integrin always contains one alpha chain and one beta chain. Both alpha and beta subunits contribute to the bindings of the cell to other cells or to the environment [2, 5].
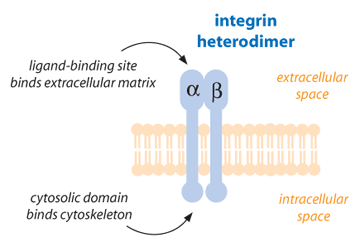
To date, 17 alpha and 8 beta subunits have been identified. From these subunits, some 24 integrins are formed in nature, which indicates that not all possible combinations necessarily exist. Integrins are able to bind to multiple partners called ligands. These ligands are mainly found in the external environment for the cells (extracellular matrix molecules). They can also be signal molecules, such as growth factors, that are released by other cells and deliver signals. Integrins are important not only in cell adhesion but also in cell movement and migration. For instance, during wound healing, epidermal cells next to the wound site migrate into the wound by changing the number, type, and distribution of integrins present in the cells [2, 6, 7].
How can integrins affect cell motility?
The integrins can only bind to their binding partners when there is a minimum number of integrins present at specific places known as focal contacts. The affinity of integrins for their ligands is not very strong. Therefore, to form effective cell-to-cell or cell-to-extracellular matrix contacts, several integrins must be localized at the focal contact. Accordingly, when the integrins are diffusely distributed over the cell surface, no strong adhesion will be present. The low affinity of integrins for their ligands is necessary to prevent irreversible binding of cells, which would result in a lack of motility. By making and breaking focal contacts, a cell can actually move through its environment [6, 8, 9].
Although the inflammatory response can make things worse, given enough time inflammation does dissipate, the reactive oxygen and nitrogen species are no longer created and the area is allowed to recover to a normal healthy state. Why, then, will the damaged neurons not send out new growth cones across the damaged area to reestablish previously severed connections?
Integrins in epidermal cells during wound healing
Human epidermal cells are able to make at least eight integrins that mediate cellular responses to various extracellular matrix molecules. Among these integrins, some are involved in wound healing [6]. Underneath the normal epidermis, there is a thin layer of cells on which the epidermal cells are laid. This layer, located on the border between the upper and lower layers of skin (epidermis and dermis), is called the basement membrane. Epidermal cells are bound to the basement membrane via specified integrins such as integrin alpha-6 beta-4 [10]. During wound repair, epidermal cells on the wound margin lose their contact with their underlying layer and start migrating on the wound bed. On the wound bed, the migrating epidermal cells encounter some new molecules (released due to the initial clot and disrupted skin at the wound site) that they normally do not encounter [11, 12]. Migrating epidermal cells still make integrin alpha-6 beta-4, but its distribution in the cell membrane is not specified to the base of the epidermal cells; instead, it is diffused all over the cell surface. This promotes the migratory characteristic of the epidermal cells [13].
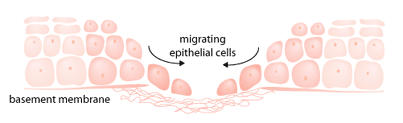
In normal epidermis, integrins alpha-2 beta-1 and alpha-3 beta-1 are involved in cell-to-cell contacts. This means that they cause epidermal cells to stick to each other [14]. Although migrating epidermal cells make higher levels of these two integrins, their function switches from cell-to-cell contacts to wound matrix molecule-binding [15]. During wound healing, migrating epithelial cells express some new types of integrin, such as integrin alpha-5 beta-1.This integrin is not present in normal unwounded epidermis. The appearance of integrin alpha-5 beta-1 starts shortly after wounding and it lasts only for a short duration. It is believed that this integrin is involved in cell interactions with molecules present in the initial clot. As the clot disappears, this integrin disappears too [6, 15].
After a while, migrating epidermal cells start dividing and proliferating in order to make epidermal cell sheets and cover the wound area. This process is called re-epithelialization 2. At the same time, the epidermal cells start producing some of the components of basement membrane in order to stabilize the structure of newly-formed epidermis. The fusion of moving epidermal sheets is associated with the production of a new integrin, called alpha-v beta-6 [16]. This integrin is not normally found in normal adult skin and mucosa [17]. Although integrin alpha-v beta-6 is a binding partner of some abundant wound matrix molecules, it is not a necessary factor for epidermal cell migration, because it appears relatively late in wound healing, when cell migration has stopped [18], 6. So, what is the main role of integrin alpha-v beta-6 in wound healing? Considering that it is expressed after the completion of wound coverage, it is possible that integrin alpha-v beta-6 is involved in reconstruction of the new basement membrane 6.
According to experiments done on cultured epidermal cells, it has been shown that integrin alpha-v beta-6 can activate a growth factor called transforming growth factor beta or TGF [19]. However, the relation between alpha-v beta-6 integrin and TGF has not yet been shown directly in animal models or in humans. TGF is secreted from different cells involved in the healing process, including epidermal cells, and has many important roles in several steps of wound healing. It is involved in the regulation of the immune response upon wounding, in the reconstruction of destroyed dermis and formation of new blood vessels, and in the production of appropriate integrins both in dermal and epidermal cells [19]. It also sends signals to epidermal cells to stop dividing when their number is sufficient; an important task that prevents scar formation [20]. The level of TGF is especially high when alpha-v beta-6 integrin is present at the wound epidermal cells (unpublished). Therefore, although this is still under investigation, it has been speculated that the role of integrin alpha-v beta-6 during wound healing is the activation of TGF . If this were true, alpha-v beta-6 integrin would be a key player in the later stages of wound healing.
Summary
The process of skin wound coverage, or re-epithelialization, is very important in that it restores the integrity of skin and mucosa and prevents infection. Integrins, which are the main cell surface proteins that attach a cell to other cells and to its environment, are important players of this process. They undergo significant changes during the epidermal cell migration and skin reconstruction stages of wound healing. Some integrins of migrating epidermal cells are re-distributed in the cell membrane (such as integrin alpha-6 beta-4), some change function (such as integrins alpha-2 beta-1 and alpha-3 beta-1) and some appear temporarily during wound repair (such as integrins alpha-5 beta-1 and alpha-v beta-6). The regulatory mechanisms and function of these integrins are not totally understood. A better understanding of the characteristics of these integrins in normal wound healing will definitely help us to better know and manage the abnormal conditions in which re-epithelialization does not occur properly (chronic non-healing wounds) or cases where there is an excessive epidermal cell proliferation (scars).
References
1. Hakkinen L, Koivisto L, Gardner H, Saarialho-Kere U, Carroll JM, Lakso M, Rauvala H, Laato M, Heino J, Larjava. Increased expression of beta6-integrin in skin leads to spontaneous development of chronic wounds. H.Am J Pathol. 2004 Jan;164 (1):229-42.
2. Hakkinen L, Uitto VJ, Larjava H. Cell biology of gingival wound healing. Periodontol. 2000 Oct; 24 (1):127-152.
3. D.M. Secko. Surface Receptors: A Biological Conduit for Information Transfer.
4. Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev. 1998 50: 197-263.
5. Rojas AI, Ahmed AR. Adhesion receptors in health and disease. Crit Rev Oral Biol Med. 1999 10 (3): 337-358.
6. Larjava H., Haapasalmi K, Salo T, Wiebe C, Uitto V-J. Keratinocyte integrins in wound healing and chronic inflammation of the human periodontium. Oral diseases 1996 2: 77-86.
7. Wehrle-Haller B, Imhof Beat A. Integrin-dependent pathologies. J Pathol. 2003; 200: 481-487.
8. Cohen M, Joester D, Geiger B, Addadi L. Spatial and temporal sequence of events in cell adhesion: from molecular recognition to focal adhesion assembly.Chembiochem. 2004 Oct 4;5 (10):1393-9.
9. Schwartz MA. Transmembrane signaling by integrins. Trends Cell Biol. 1992; 2: 304-308.
10. Stepp MA, Spurr-Michaud S, Tisdale A. alpha-6 beta-4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad sci USA 1990; 87: 8970-8974.
11. Gailit J, Clark RAF. Wound repair in the context of extracellular matrix. Curr Opin Cell Biol 1994; 6: 717-725.
12. Stenn KS, Malhotra R. Epithelialization. In: IK Cohe, RF Diegelmann, WJ Lindblad, eds. Wound healing. Biochemical & clinical aspects. WB Saunders Co: Philadelphia, pp 115-127.
13. Kurpakus MA, Quaranta V, Jones JCR. Surface relocation of aopha6 beta4 integrins an assembly of hemidesmosomes in an in vitro model of wound healing. J Cell Biol 1991; 115: 1737-1750.
14. Uttito J. Christiano AM. Molecular genetics of the cutaneous basement membrane zone. Perspectives in epidermolysis bullosa and other blistering skin diseases. J Clin Invest. 1992; 90: 687-692.
15. Larjava H, Salo T, Haapasalmi K. Expression of integrins and basemant membrane components by wound keratinocytes. J Clin Invest. 1993; 92: 1425-1435.
16. Haapasalmi K, Zhang K, Tonnesen M. Keratinocytes in human wound express alpha-v beta-6 integrin. J Invest Dermatol. 1996; 106: 1-7.
17. Breuss JM, Gillett N, Lu L, Sheppard D, Pytela R. Restricted distribution of integrin beta 6 mRNA in primate epithelial tissues. J Histochem Cytochem 1993; 148 (5): 1407-21.
18. Koivisto L, Larjava H, Hakkinen L, Uttito VJ, Heino J, Larjava H. Different integrins mediate cell spreading, haptotaxsis and lateral migration of HaCaT keratinocytes on fibronectin. Cell Adhes Commun 1999; 7: 245-257.
19. Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alpha-v beta-6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004 Jun 7;165 (5):723-34.
20. Glick AB, Kulkarni AB, Tennenbaum T, Hennings H, Flanders KC, O’Reilly M, Sporn MB, Karlsson S, Yuspa SH. Loss of expression of transforming growth factor beta in skin and skin tumor is associated with hyperproliferation and a high risk for malignant conversion. Proc Natl Acad Sci USA 1993; 90(13): 6076-80.
(Art by Jen Philpot)