ISLET AMYLOID – A CULPRIT IN TYPE 2 DIABETES
It is estimated that nearly 194 million people worldwide have diabetes. This is an increase from the 1995 global estimation of 135 million which was published in a World Health Organization study in 1998[1]. The International Diabetes Federation reconfirms that type 2 diabetes, which is the non-insulin dependent type, constitutes about 85% to 95% of all diabetes cases in developed nations and accounts for an even higher percentage in developing nations. Diabetes continues to affect increasing numbers of people around the world while public awareness remains low.
What is type 2 diabetes?
Type 2 diabetes is a disease often associated with growing obesity in the adult population. More recently, changes in diet and reduced physical activity are probable contributors to the increased occurrence of this disease in children[2]. Type 2 diabetes is characterized by elevated blood glucose levels due to problems with the secretion of insulin and its action within the body. Over time, the blood glucose levels increase while insulin secretion steadily decreases. This is primarily due to the gradual damage to insulin-producing beta cells in the pancreas by the formation of toxic protein-containing deposits within the pancreatic islets. At autopsy, these deposits, which replace the endocrine cells within the islet, have been demonstrated in up to 90% of individuals with type 2 diabetes. These toxic deposits are now recognized as a pathological feature of this disease[4].
What is Amyloid?
Amyloid describes a deposition of protein generally located on the outside of a cell. The name amyloid comes from the latin word amylum which means starch. Initially scientists thought that the deposits were starch-like but later discovered that they were actually protein-like. Amyloid deposits can be classified into systemic amyloidosis where deposits are present in many organs, or local amyloidosis where deposits are confined to a specific tissue.
Discovery of Amyloid.
100 years ago a pathologist, Eugene Opie, described the occurrence of ‘hyaline degeneration of the islets of Langerhans’ in patients with hyperglycemia[2] and, not knowing that these pancreatic islets contained the insulin producing cells, suspected that there may be a relationship between the presence of these hyaline deposits and the development of diabetes. Later, the hyaline degeneration was described as amyloid deposit.
Amyloid and Disease.
Local amyloidosis is generally related to aging diseases. The two best examples of localized amyloidosis are Alzheimer’s disease and type 2 diabetes mellitus (DM2). Localized amyloid deposition results from the production of a unique polypeptide, which contains an amyloidogenic sequence and is capable of forming a beta-pleated sheet structure necessary for these deposits to aggregate. In Alzheimer’s the unique peptide is the beta-amyloid protein (Abeta) and in type 2 diabetes it is the islet amyloid polypeptide (IAPP) also known as amylin[4]. In order for fibrils to form in neurons and in pancreatic islets, in Alzheimer’s and DM2 respectively, the unique peptides must be present.
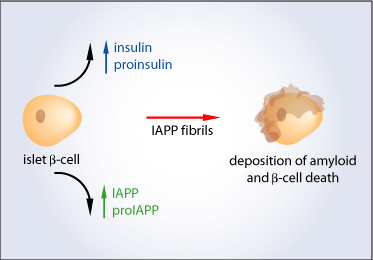
Islet amyloid and type 2 diabetes – is there a relationship?
Over the years, the major peptide component of amyloid was difficult to identify due the extreme insolubility of the deposits. However, the peptide was finally identified by Per Westermark in 1986. The initial studies were performed on insulinoma tissues derived from insulin-producing tumors in pancre-atic islet b-cells. The studies resulted in the identification of the peptide known today as islet amyloid polypeptide (IAPP)[4]. IAPP is an important player in the formation of amyloid deposits and the progression of type 2 diabetes.
IAPP is a 37 amino acid peptide which is co-stored and co-secreted with insulin in a molar ratio of approximately 1:100 (IAPP: insulin) from the beta-cell secretory granules of the pancreatic islets. IAPP is derived from a larger molecule called proIAPP and processed by two enzymes called prohormone convertases (PC) – PC 1/3 and PC2, which are also responsible for the conversion of proinsulin to insulin[3].
In type 2 diabetes, the proinsulin to insulin conversion is impaired due to a processing defect in the beta-cells. It is thought that since IAPP and insulin are co-stored and co-secreted they likely use similar pathways; it is possible that the IAPP processing pathway is also impaired in cells affected by type 2 diabetes. This impairment may lead to the accumulation of an incompletely cut precursor molecule, proIAPP, within the beta-cells. Since proIAPP also contains the amyloidogenic portion of the mature human IAPP, it may aggregate within the beta-cells, ultimately contributing to cell death. This suggests that islet amyloid deposition is an early event in the pathogenesis of type 2 diabetes and that it is associated with a reduction in insulin release well before the onset of hyperglycemia[3,4,5].
hIAPP transgenic mice develop amyloid deposits.
Initial in vivo models for islet amyloidosis were difficult to establish. Although the IAPP amino acid sequence is conserved between species, only humans, non human primates and cats express a form of IAPP that is able to form fibrils. Mice and rats, the standard animals used for laboratory studies, produce IAPP that does not form the fibrils because it lacks the proline amino acid residue necessary for the formation of the beta-pleated sheet. For this reason, scientists were unable to study the function of the human amyloidogenic form of IAPP. Recently, with the use of transgenic mouse technology, scientists were able to study the effects of human IAPP overproduction in an obese diabetic mouse model. The expression of the IAPP gene is specifically targeted to islet beta-cells so that hIAPP is only expressed in these cells. Mice that develop diabetes spontane-ously show higher blood glucose levels and lower insulin levels as well as the presence of amyloid deposits in their beta-cells. This suggests that the high fat diet leading to the obesity of these mice may be partly responsible for the development of both an IAPP and an insulin processing defect that could lead to the accumulation of amyloid deposits in the beta-cells[6].

Other roles for IAPP?
The known functions of IAPP are related to glucose metabolism: IAPP suppresses insulin-mediated glucose uptake in skeletal muscle and prevents the release of insulin with glucose stimulation. Other suggested roles include regulation of renal filtration, calcium homeostasis and vasodilation. Although a definite function for IAPP has not been clearly indicated, its role in islet amyloid formation in the pancreas of individuals with type 2 diabetes is clear – it has a role in the pathogenesis of the islet beta-cell dysfunction[3].
Targeting amyloid for therapy.
There is evidence to suggest that aggregation of the IAPP fibrils plays an important role in beta-cell death and the progression of type 2 diabetes. To prevent amyloid accumulation, the following are possible therapeutic targets:
1. reducing IAPP release from the islet to decrease the precursor pool of available amy-loidogenic IAPP
2. using anti-diabetic agents that act by decreasing IAPP release by b-cells eg. met-formin, thiazolidinediones
3. development of inhibitors to target the amyloidogenic region of the IAPP molecule using synthetic peptides
Summary.
Islet amyloidosis is both a consequence and a cause of type 2 diabetes. It is induced by insulin resistance, stimulating not only insulin but IAPP secretion, and contributes to insufficient insulin in the body by promoting b-cell failure. In this respect, IAPP and islet amyloid provide an attractive target for diabetes therapy.
References.
1) http://www.idf.org/home/index.cfm?node=264
2) Hoppener JW, Nieuwenhuis MG, Vroom TM, Ahren B, Lips CJ. (2002) Role of islet amyloid in type 2 diabetes mellitus: consequence or cause? Mol Cell Endocrinol. Nov 29; 197(1-2):205-12.
3) Hull RL, Westermark GT, Westermark P, Kahn SE. (2004) Islet amyloid: a critical entity in the pathogene-sis of type 2 diabetes. J Clin Endocrinol Metab. Aug;89(8):3629-43.
4) Kahn SE, Andrikopoulos S, Verchere CB. (1999) Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. Feb; 48(2):241-53.
5) Clark A, Nilsson MR. (2004) Islet amyloid: a complication of islet dysfunction or an aetiological factor in Type 2 diabetes? Diabetologia. 2004 Feb;47(2):157-69. Epub 2004 Jan 13.
6) Porte D Jr, Kahn SE. (2001) beta-cell dysfunction and failure in type 2 diabetes: potential mechanisms. Diabetes. Feb;50 Suppl 1:S160-3.
Glossary.
Amyloidosis – A disorder that results from the abnormal deposition of a particular protein, called amyloid, in various tissues of the body. Amyloid protein can be deposited in a localized area and not be harmful, or it can cause serious changes in virtually any organ of the body.
Beta cell – A type of cell in the pancreas. Within the pancreas, the beta cells are located in areas called the islets of Langerhans where they constitute the predominant type of cell. The beta cells make and release insulin, a hormone that controls the level of glucose (sugar) in the blood.
Endocrine – Pertaining to hormones and the glands that make and secrete them into the blood-stream through which they travel to affect distant organs eg. Islets of Langerhans in the pancreas, which secrete insulin.
Hyperglycemia – An elevated level specifically of the sugar glucose in the blood. Often found in diabetes mellitus. It occurs when the body does not have enough insulin or cannot use the insulin it has to turn glucose into energy. The signs of hyperglycemia are polydipsia (a great thirst), polyuria (a need to urinate often), and a dry mouth. The term “hyperglycemia” comes from the Greek “hyper-” = high, over, beyond, above + “glykys” = sweet + “haima” = blood. High sweetness (sugar) in the blood.
Islets of Langerhans – Known as the insulin-producing tissue, the islets of Langerhans do more than that. They are groups of specialized cells in the pancreas that make and secrete hormones. Named after the German pathologist Paul Langerhans (1847-1888), who discovered them in 1869, these cells sit in groups that Langerhans likened to little islands in the pancreas. There are five types of cells in an islet: alpha cells that make glucagon, which raises the level of glucose (sugar) in the blood; beta cells that make insulin; delta cells that make somatostatin which inhibits the release of numerous other hormones in the body; and PP cells and D1 cells, about which little is known. Degeneration of the insulin-producing beta cells is the main cause of type I (insulin-dependent) diabetes mellitus.
Pancreatic beta cell – A type of cell in the pancreas that makes insulin. The pancreas is a fish-shaped organ that stretches across the back of the abdomen behind the stomach. Within the pan-creas there are areas that are called the islets of Langerhans. The beta cells constitute the pre-dominant type of cell in the islets.
(Art by Jiang Long – note that high res versions of image files available here)