CHARACTERIZATION OF KASHA-MEDIATED SUSCEPTIBILITY AND HISTOPATHOLOGICAL HALLMARKS OF EARLY-ONSET CHEWHEIMER’S DISEASE
Don’t forget, this paper is part of a collection of Wookiee scientific reports that will be published in the print edition of the SCQ. More details can be found here.
Annals of Praetachoral Mechanics. (2014). Vol 1. pp71-88 pdf download
ABSTRACT
Since the rise of Force-vector injections (FVIs), the incidence of early-onset CD (eCD) has escalated significantly, with a large impact on quality of life in the wookiee community. However, while a large number of wookiees became afflicted with eCD, a small number remained healthy. This study first aimed to discover any genetic discrepancies between wookiees who developed eCD following FVI and those who did not to uncover a potential risk factor for the disease. The second aim was to characterize the hallmarks of eCD and compare them to the related human form, Alzheimer’s disease (AD). Significant differences were discovered in eCD prevalence post-FVI based on allelic variations at the Kashyk gene, which codes for a lipoprotein, highly similar to the human apolipoprotein. Furthermore, similar patterns of pathological hallmarks in wookiee eCD brains when compared to human AD brains were identified, via PET-PiB scan, cerebrospinal fluid (CSF) Aβ levels and post-mortem studies. Together, these results shed light on the importance of genetic screening for Kashyk prior to FVI, as well as set the stage for future development of therapies in parallel with human AD.
Keywords: early-onset Chewheimer’s Disease, Kashyk genetic screening, PET-PiB imaging; amyloid plaque and neurofibrillary tangle imaging
Introduction
Following the successful transplantation of wookiee midi-clorians containing mutations at positions 263 and 312012 into oocytes and in vitro fertilization of female wookiees [1]; multiple attempts have been made to incorporate these mutations into adult wookie midi-clorians, most of them failing. [2, 3] Recent work by Yoda et al. [4] resulted in the first successful and functional Force-able wookiees, where a previously characterized midi-clorian retrovirus, Darth TMS of the family Tenebrousviridae, was utilized to permanently incorporate the mutations in all the adult wookie midi-clorians. However, many of the Force-vector injections (FVIs) resulted in fatally ill and mentally unstable wookiees, putting a halt in the progress of the field. Early work has shown that the pathogenesis of the disease that developed post-FVI greatly resembles Chewheimer’s disease, but with a very rapid onset and progression, and was therefore termed early-onset Chewheimer’s disease (eCD). [5]
Chewheimer’s disease is named after the first individual characterized with the condition, Chewbacca, in 2 ABY, in combination with the human form, Alzheimer’s disease (AD) [6]. Much like the human condition, CD is a progressive neurodegenerative disease.[6] In wookiees, it is characterized by rapid memory loss, personality changes such as loss of loyalty, physical weakness, apathy (loss of the characteristic short temper) and language deficits (inability to roar). [6] These symptoms are a result of progressive grey matter loss starting in the limbic system of the brain and extending to the cortex. [5] 10 years ago, this disease affected roughly 10,000 wookiees in Kashyyyk.[7] However, this number has risen substantially in the past few years, with a current estimate of 300,000 affected and counting. [7]
The rise in CD prevalence (in particular, its early-onset form, eCD) is thought to be a direct result of increased popularity of FVIs. [8] A survey by Calrissian et al. (20 ABY) revealed that since introduction of FVIs, more than 500,000 wookiees have chosen to undergo the procedure. [8] This high number is thought to be due to the well-known loyalty of the wookie species, as well as their eagerness to assist in the rebellion. [8]
Since the introduction of the FVI in the wookiee population, roughly 60 percent of users have become afflicted with eCD over the course of only several months, while the other 40 percent remained healthy. [7,8] The discrepancy in disease appearance amongst FVI users has been an area of intense investigation, and points to a likely genetic predisposition in those afflicted with eCD following FVI. [7,8]
While some genetic studies have been carried out, they have been unable to identify a direct genetic risk factor for eCD. [9,10] Calrissian (21 ABY) provided evidence for allelic variations at various loci coding for apoptotic genes responsible for neuronal death in an animal model of eCD (Anooba Tatooinis). [9] In addition, in the human AD, allelic variations at the APOE gene have been implicated as a risk factor. [10] Specifically, of the three allelic forms, APOE2, 3, and 4, individuals with the APOE4 allele have a higher likelihood of developing AD. [10] Whether this or a related gene also plays a role in eCD has not yet been investigated.
In addition to any genetic variations, the histopathological changes underlying eCD remain to be elucidated. In humans, AD pathogenesis has been highly examined, and is known to present itself with two hallmarks: amyloid plaques, consisting primarily of insoluble amyloid β (Aβ) peptides deposited between neurons, and neurofibrillary tangles (NFTs), composed of intraneuronal hyperphosphorylated tau aggregates. [10,11] Whether these hallmarks also exist in wookiees is relatively unknown, with some early evidence for β-pleated amyloid sheets and tau aggregates in post-mortem wookiee brains. [12] Comparing eCD to AD is useful as wookiees, arboreal humanoid mammals, share many genetic similarities with humans, which oftentimes translate to comparable disease courses (for example: asthma, and Blackwing virus). [13,14] Furthermore, previous studies have already found that the processes underlying eCD oxidative damage and neurodegeneration parallel those of AD. [15] In humans, there are techniques available to screen for some hallmarks of AD. First of all, previous work has shown that patients with AD have less detectable Aβ in their CSF, likely because this Aβ is forming amyloid plaques in the brain and not entering the CSF as a result. [10] Secondly, levels of cortical fibrillar amyloid plaques are higher in the cortices of AD-afflicted individuals, which can be measured via positron emission tomography (PET) scan using the imaging agent Pittsburgh compound B (PiB). [10]
The purpose of this study was to investigate the genetic link between force acquisition via FVIs and the risk of developing eCD, as well as observe histopathological changes underlying eCD in comparison to the human AD. We hypothesized that predisposition to eCD derives from allelic variations in affected versus unaffected wookiees, and that the pathological hallmarks of eCD are highly comparable to the human AD.
Materials and Methods
Blood Samples.
All studies were approved by the Jedi Council Research Ethics Board (JCREB). We collected blood samples from 4742 eCD-affected wookiees (following FVI), 15821 healthy, FVI wookiees and 301734 normal wookiees that did not receive FVI. Additional blood samples were provided from the Chewbacca Disease Sequencing Project database (CDSP) from 9134 deceased late-onset CD and 20026 deceased eCD individuals. Of these individuals, 700 were part of another study at the Chewheimer’s Disease Research Center (CDRC) at the University of Naboo. 500 of these subjects were recruited for our CSF Aβ and PiB-PET studies.
Extraction and Isolation of genomic DNA.
Rapid extraction of high quality genomic DNA from the blood samples collected was conducted as previously described [16]. Briefly, blood cells were lysed using the lysis buffer prepared as described in the protocol and haemoglobin was removed. The DNA was isolated and purified following steps involving nucleic lysis buffer, saturated NaCl/chloroform and ethanol.
SNaPshot analysis for Kashyk isoform detection.
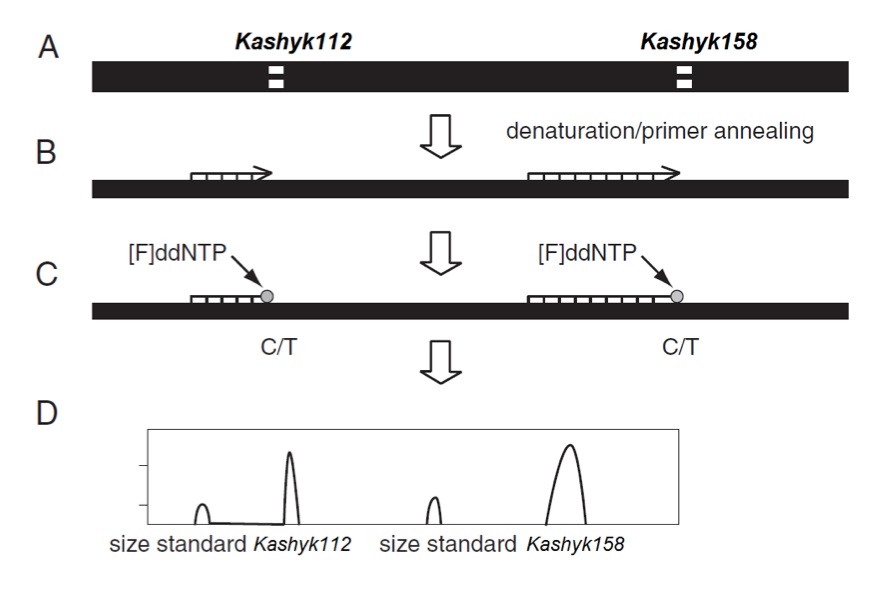
The Kashyk isoforms in each sample were detected utilizing a previously described method for APOE genotyping. (Alternate protocol 3; [17]) Initially a 217bp fragment (See sequence alignment Figure 2) containing both Kashyk single nucleotide polymorphism (SNP) sites of interest were amplified by PCR. The PCR products were then used as templates for a minisequencing reaction utilizing dideoxynucleotides labeled with different fluorophores that terminate the elongation at the polymorphic SNP sites. The GalacticGeneScan software was used to analyze the data and identify the Kashyk genotypes. Primers designed for the SNaPshot PCR step (CL5938 lot; Kamino Cloning Reagents, Outer Rim):
Forward: 5’-CCA AGG AGC TGC AGG CGG CGC A-3’ (before Kashyk112)
Reverse: 5’-GCC CCG GCC TGG TAG ACT GCC A-3’ (after Kashyk158)
Figure 1 (Click to Enlarge) Kashyk genotyping using the SNaPshot method. (A) The initial PCR results in a 217-bp fragment, which encompasses both the Kashyk112 and Kashyk158 loci, (B) In a minisquencing step, primers ending one base pair 5′ of the Kashyk112 and Kashyk158 polymorphic sites are mixed in one reaction. (C) Incorporation of fluorescently labeled ddNTP is followed by (D) separation of the labeled primers on the Yoda Genetic Analyzer capillary system (Kamino Cloning Reagents, Outer Rim).
Figure 2 (Click to Enlarge) Human APOEe2 (cys112, cys158) and Wookie KashC (cys112, cys158) gene sequence alignment – CLUSTAL O (1.2.1).
Subjects.
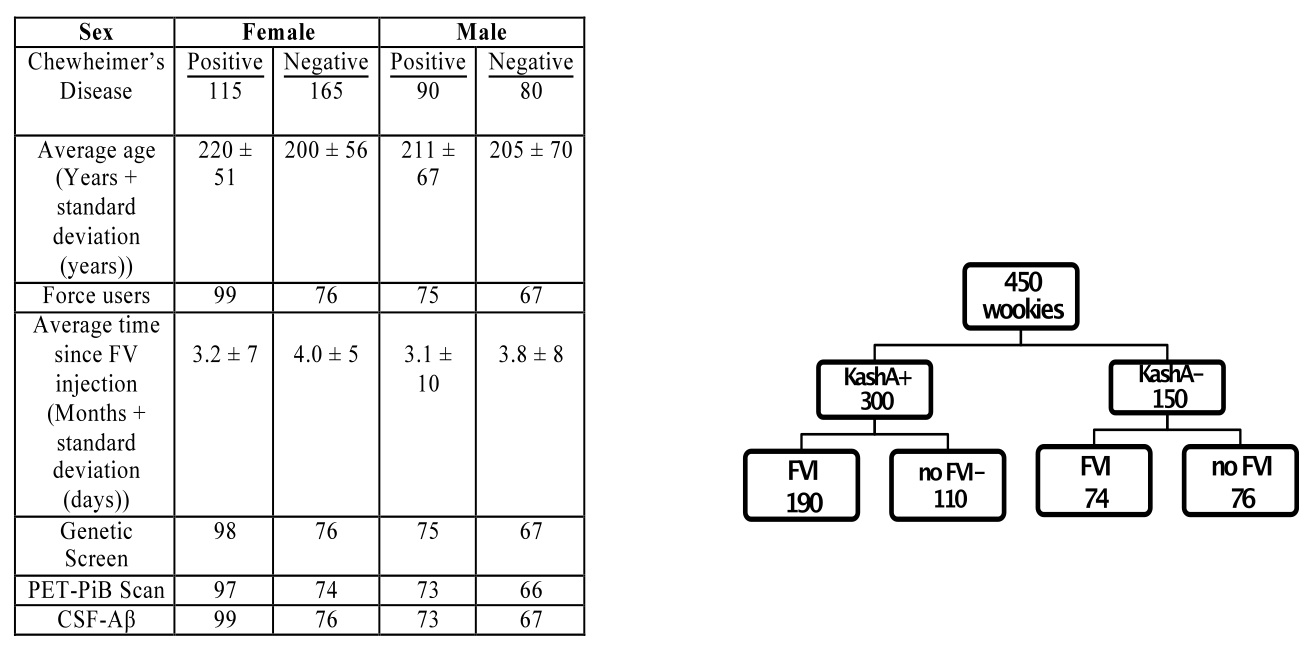
500 wookiees were recruited from the early Chewheimer’s Disease Research Center (CDRC) at the University of Naboo. Of those, 312 were female, and 188 were male. The average age of participants was 209±61 years. Wookiees underwent a survey on the history of force use, overall health, as well as the Tatooine University mental status exam in person. Those with pre-existing health problems (cardiovascular disease, midi-chlorian deficiency, Wookiee rabies) were excluded from the study to reduce confounding factors. This resulted in a total of 280 females and 170 males. Of 280 females, 115 had a previous diagnosis of eCD, and 165 were healthy. Of 170 males, 90 had eCD, and 80 were healthy. In the female cohort, 175 had received FVIs, and 99 of those had eCD, while 76 of them were healthy. Of the ECD-positive males, 75 had received FVIs, while 67 healthy males had the injection. Further details of the breakdown of participants can be found in Table 1. Participants were age and sex matched throughout our study.
Table 1 (Click to Enlarge) (left) Breakdown of participants for the PET-PiB and CSF-Aβ. (right) Layout of initial experimental design and grouping. Groups were further subdivided based on age and sex.
Cerebrospinal fluid (CSF) collection, processing, and biomarker measurement.
CSF collection and enzyme-linked immunosorbant assay (ELISA) were performed as previously described. [10] Via lumbar puncture, CSF was collected in polypropylene tubes at 8:30 AM after overnight fasting as described previously. [18] To avoid gradient effects, samples were gently inverted, centrifuged quickly at low speed to pellet cellular elements, and aliquoted at 500μl into polypropylene tubes. Samples were then frozen at −84°C. Aβ analysis was completed using commercial ELISA (Boba Fett Biotech, Mandalore, The Galaxy Far Far Away). Samples were kept on ice at all times prior to assaying.
PET-PiB imaging.
PET studies were carried out in collaboration with the PET facility at the University of Naboo and based on a previously described protocol. [10] In vivo amyloid imaging via positron emission tomography (PET) with PIB ([N-methyl-[11C]]2-(4′-methylaminophe-nyl)-6 hydroxybenzothiazole) was performed as described previously. [19] 12 mCi of [11C]PIB was administered intravenously simultaneous with initiation of a 60-minute dynamic PET scan in three-dimensional mode, and dynamic PET images were reconstructed. Consequently, three-dimensional regions-of-interest were created for each participant based on their individual MRI scans (T1-weighted 1×1×1.25mm MPRAGE). Each region of interest had a calculated binding potential (BP), to characterize regional binding values proportionally to number of binding sites. BP values from prefrontal cortex regions of interest were averaged to calculate a mean cortical binding potential (MCBP) value based on brain regions known to have high PIB uptake among participants with eCD. [19] This derived MCBP value has been shown to correlate inversely with CSF Aβ, and predict the progression from cognitively healthy to symptomatic eCD. [18,19]
Post-Mortem Studies.
We collected 50 post-mortem brains of eCD-afflicted individuals. eCD clinical diagnosis had been performed using standard criteria (CDRC). The mean Tatooine University mental status exam score was 9±3. Mean age was 290±20 years and mean duration of disease was 80±15 years. The 50 subjects lacked other brain pathologies. The superior frontal and medial temporal cortices of the brain were dissected at autopsy (as previously described). [11] Following autopsy, these brain regions were immersed in cold 4% paraformaldehyde for 48h, cryoprotected in sucrose and sectioned at 40 microns using a freezing sliding microtome. Tissue sections were then serially collected in a cryoprotectant solution and stored at -80°C until analysis.
Histology.
The presence of amyloid plaques and NFTs are characteristics of the human AD, and are thought to play a role in pathogenesis. [10,11] To determine whether similar hallmarks occur in eCD, we used immunohistochemistry as previously described modified for the wookiee species. [11,12] Tissue sections from the CDRC eCD autopsy group in 4% paraformaldehyde fixed were washed in PB, incubated in 70% formic acid (FA) and re-washed in PB. Subsequently, sections were rinsed in TBST and incubated in 3% normal serum in TBST for 30min. Sections were then incubated overnight at 4°C in monoclonal 6E10 (Aβ1-16) antibody. The primary antibody used for NFT detection was phospho-tau AT8. After washing in 1% normal serum in TBST, sections were incubated for 1h in the species-appropriate IgG (Vader Vector Laboratories, Death Star, The Galaxy Far Far Away). The sections were then washed in TBST and processed with an Elite kit (Vader Vector Labs) in TBST for 30min at room temperature. Following imidazole acetate buffer (IAB) washes, a solution containing 0.06% 3’,3-diaminobenzidine tetra-hydrochloride (DAB) / 0.0016% H2O2 was added for 5min. Following, sections were washed in IAB, mounted on slides, dehydrated in ethanol, cleared in xylene and coverslipped.
Timing of Assessments.
Clinical assessment was within ±3.0 months (SD=2.0 months) of the PET scan and ±2.9 months (SD=2.5 months) of the CSF assessment.
Statistical Analyses.
All analyses were conducted using Sith Statistics System 17.0 (Korriban, The Galaxy Far Far Away). We tested for group differences (i.e., FVI, Kashyk status) using Student’s t tests for continuous variables and Fisher’s exact test for dichotomous variables. We used a hierarchical multiple regression model to examine FVI×Kashyk status interaction for each of the separate estimates of amyloid deposition. Each complete cohort had a separate regression model (i.e., MCBP and CSF Aβ). All statistical significance tests were 1-tailed, and α=0.05.
Results
Wookie APOE analogue: Kashyk
Based on the nature of the symptoms observed in both rapidly progressing eCD and late-onset CD the wookie genome was screened for targets with high sequence identity with genes involved in the corresponding human disease, AD. A Basic Local Alignment Search Tool (BLAST) search of gene sequences from the Wookie Genome Project (WoGenPro) database and NCBI GenBank yielded multiple hits. The search was narrowed down to genes known to be involved in human AD, such as APOE, by removing all unrelated sequences from the search. One of the genes identified through this search was Kashyk, a wookie-specific protein, which was been previously studied due to its involvement in various diseases. The BLAST search identified high local sequence similarity between APOE and Kashyk throughout the alignment of the genes; it located highly conserved regions with 99-100% similarity, as well as many regions with 73-89% sequence identity. (Data not shown) A CLUSTAL O (1.2.1) sequence alignment of the human APOE2 and wookie KashC, seen in Figure 2, reveals high sequence similarity, areas of deletion, insertion and single nucleotide differences between the sequences of the two species.
Kashyk genotyping
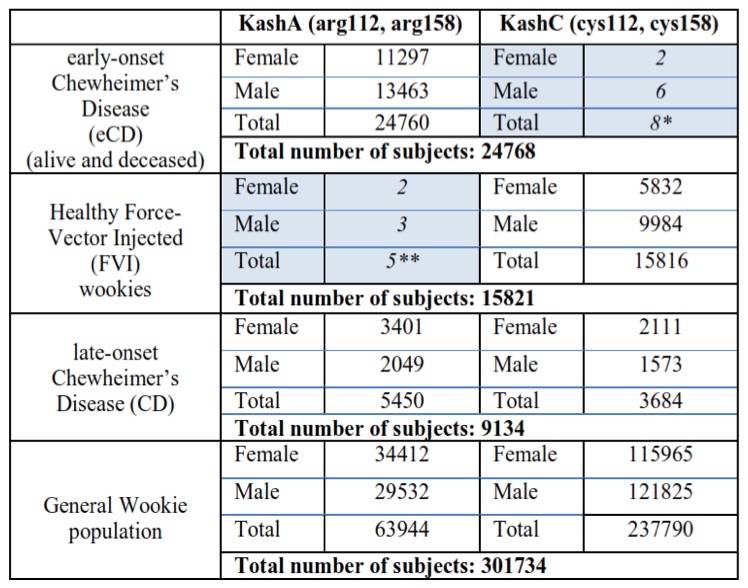
Genotype experiments revealed the existence of two Kashyk isoforms that are analogous to the human APOE2 and APOE4 proteins. Kashyk A (KashA) has arginine residues at positions 112 and 158 of the amino acid sequence, while Kashyk C (KashC) has cysteine residues at the same positions. Almost all of the eCD samples that were genotyped appeared to be KashA+ (homozygote or heterozygote carriers); similarly the majority of the healthy FVI wookiees were KashC+ (homozygote carriers). In the late onset CD group we identified carriers of both KashA and KashC with an approximate ratio of 3:2, respectively. Furthermore, measurements revealed that one fifth of the general wookie population is KashA+, with most of them being KashA/KashC heterozygotes. (Table 2)
Table 2 (Click to Enlarge) Kashyk genotyping in wookie populations
*Possibly misdiagnosed CD patients (see Discussion)
**Isolated incidents that can be traced back to a defective viral vector batch that did not include the mdDNA mutations.
CSF Aβ Cohort
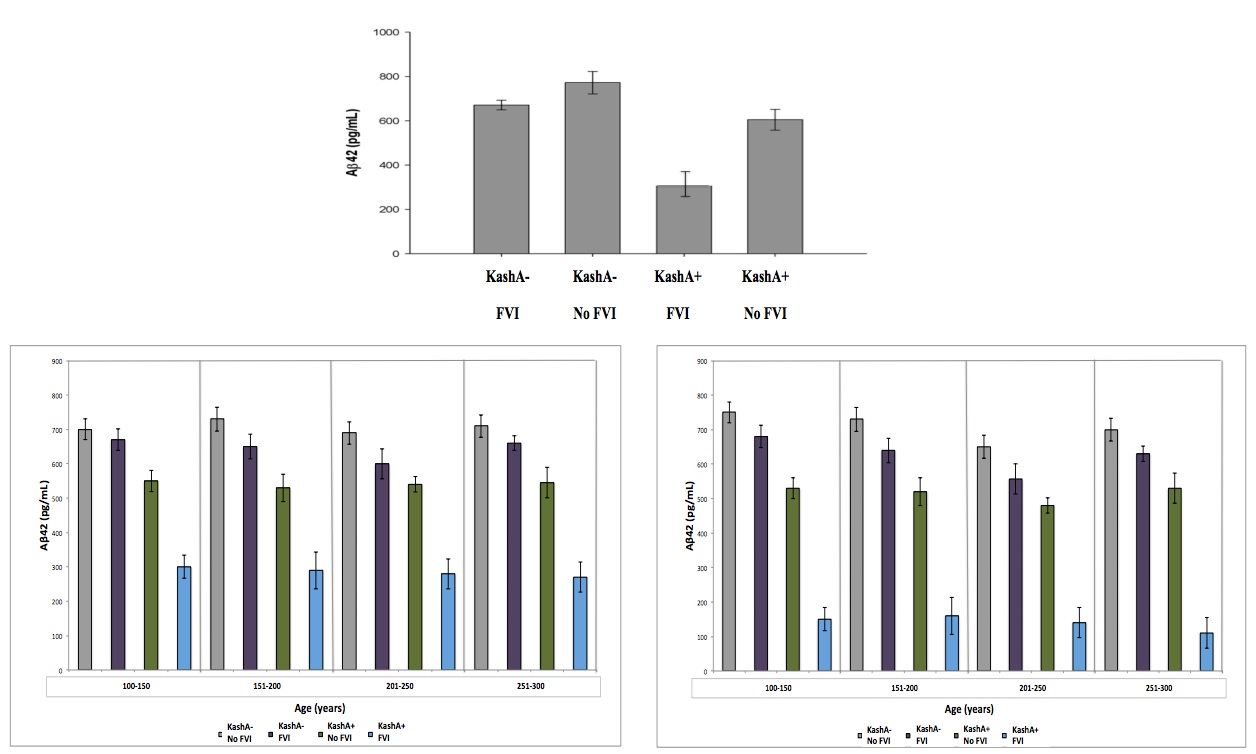
The mean Tatooine University mental status exam score for the subjects that underwent CSF Aβ measurements was 10±2. In our regression model of CSF Aβ, there were significant FVI group and KashA status differences (p=0.003) (Figure 3). Individuals without FVIs showed higher CSF Aβ levels when compared to FVI-positive individuals (p=0.002, mean difference=97.0; 95% Confidence Interval (CI)=17.34; 177.87). Also, KashA+ individuals evidenced lower CSF Aβ compared to KashA− individuals (p=0.0024, mean difference=−160.10; 95% CI=− 217.88; −89.55). Of note, there was also a significant difference in sex, with females in the
KashA+/FVI group exhibiting lower CSF Aβ levels than males in the same category (p=0.02, mean difference=92.0; 95% CI=19.50; 169.97)) (Figure 3). When categorized into age groups, there were no significant differences in CSF Aβ levels due to age (p=0.08) (Figure 3)
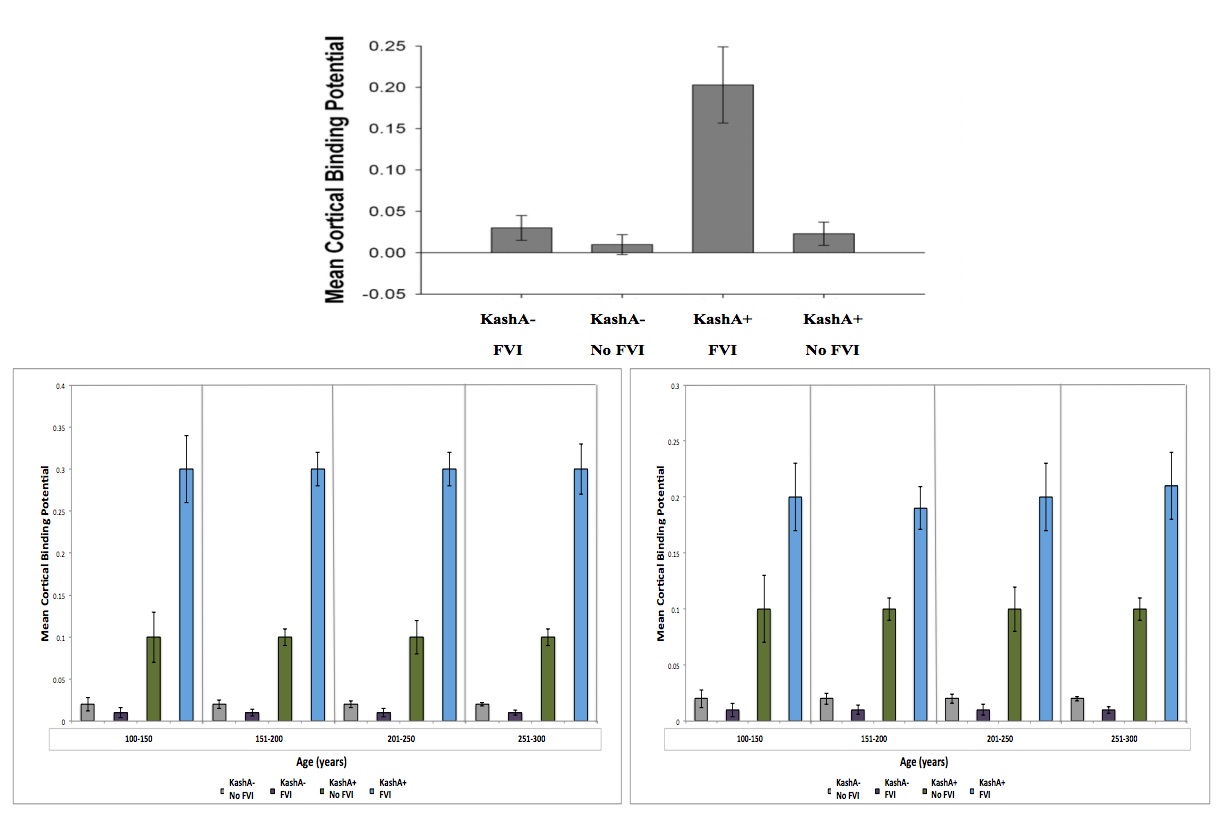
Figure 3 (Click to Enlarge) (top) Association between Kash status and FVI for CSF Aβ. Kash A carriers demonstrated lower CSF Aβ. The Kash status x FVI interaction was significant (modified from Head et al. 2012). Data was further broken down based on sex (left – male) (right – female) and into 4 age groups to account for differences due to sex/age. There was a significant different due to sex, but not due to age. See text for details. Grey: KashA-/No FVI, Purple: KashA-/FVI, Green: KashA+/No FVI, Blue: KashA+/FVI.
Interestingly, results were similar when other potentially confounding variables (i.e. education, diabetes, history of depression) were included as covariates. We used a study by Head et al. (2012) to compare eCD conditions to AD with respect to KashA and APOE status. Following comparison, we found that CSF Aβ levels followed similar trends in humans and wookiees, with lower levels in APOE4+ humans.
PET-PiB Cohort
The mean Tatooine University mental status exam score for the subjects that underwent PET-PiB measurements was 9±3. In our regression model of MCBP, there were significant FVI and APOE status differences (p=0.004) (Figure 4). FVI-positive individuals showed higher MCBP levels compared to FVI-negative individuals (p=0.0024, Mean difference=−.080; 95%CI=−.134; −.024).
Furthermore, KashA+ individuals evidenced higher MCBP compared to KashA− individuals (p=0.0026, Mean difference=.152; 95%CI=.072; .312). There was a significant FVI group x KashA status interaction (p=0.023), as well as a significant FVI effect on MCBP in KashA+ individuals compared to KashA- individuals (p=0.014, mean difference between groups=.153; 95%CI=−.218; −.069). Of note, there was a significant difference in sex, with females in the KashA+/FVI group exhibiting higher MCBP levels than males in the same category (p=0.02, mean difference=.123; 95%CI=−.258; −.049) (Figure 4). When categorized into age groups, there were no significant differences in MCBP levels (p=0.08) (Figure 4).
Figure 4 (Click to Enlarge) (A) Association between Kash status and FVI for MCBP. Kash A carriers demonstrated higher MCBP. The Kash status × FVI interaction was significant. (Modified from Head et al. 2012) Data was further broken down based on sex ((B) – male, (C) – female) and into 4 age groups, to account for differences due to sex/age. There was a significant difference due to sex, but not due to age. See text for details. Grey: KashA-/No FVI, Purple: KashA-/FVI, Green: KashA+/No FVI, blue: KashA+/FVI.
In concordance with our CSF Aβ data, results were similar when other potentially confounding variables (i.e. diabetes, history of depression) were included as covariated. Using the study by Head et al. (2012) to compare eCD conditions to AD with respect to APOE status, we once again found similar interspecies trends, with APOEε4+ individuals showing greater MCBP than ε4- individuals.
Post-Mortem Studies
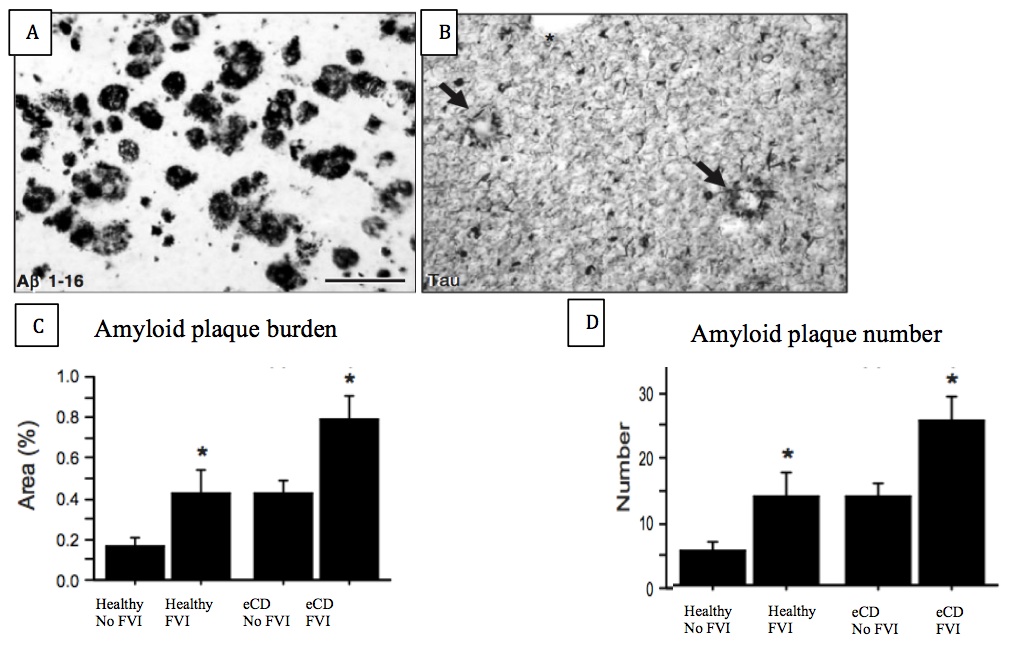
Of 50 post-mortem brains examined, 30 had a previous diagnosis of eCD, while 20 were healthy. 18 eCD individuals were previous FVI users, while 10 of the healthy individuals used FVIs. Using the Aβ1-16 antibody, we demonstrated evidence for amyloid plaque deposits in 27 out of the 30 post-mortem eCD brains examined, while none of the healthy brains exhibited these hallmarks (Figure 5A). Both plaque burden and plaque number in the superior frontal and medial temporal cortices were significantly increased in these brains (unpaired t tests, P<0.05) (Figure 5C and D). The same trends were observed for NFTs (Figure 5B). Interestingly, the brains of FVI users showed enhanced amyloid plaques and NFTs, in particular in those with a previous diagnosis of eCD.
Figure 5 (Click to Enlarge) (A) Expression of amyloid plaques and (B) neurofibrillary tangles in the wookiee cortex. (Modified from Ikonomovic et al., 2008) (C) amyloid plaque burden and (D) amyloid plaque number in the 4 examined groups. Scale bar = 175 mm
Discussion
To our knowledge, this is the first study identifying a genetic risk factor for the development of eCD following FVI, and pathological hallmarks of eCD.
This study revealed Kashyk, an APOE analogue, as the most promising candidate for eCD predisposition following FVI. In the wookie central nervous system, it is mainly produced by the deathstar cells, which are equivalent to human astrocyte cells. Kashyk is involved in regulating neuronal firing in brain regions responsible for sound vocalization, as well as co-localization of Chew Precursor Protein (CPP) and lightsaber secretase (l-secretase). [20] Mutations in different regions of this protein have been correlated with speech and cognitive impairment in diseases such as Wootism [20] and Wookielexia [21]. Experiments revealed that Kashyk is a polymorphic protein that has at least two major isoforms. KashA has arginine residues, while KashC has cysteine residues, at positions 112 and 158 of the amino acid sequence. Although these allelic forms only differ in two amino acid positions, these small differences possibly confer alterations in the protein structure and function, as they do it the case of APOE2 and APOE4. [22]
Most eCD samples were KashA+, possibly suggesting complete penetrance of the gene isoform with the disease. The small number of KashC homozygotes in the eCD population could be due to misdiagnosis of late onset CD as eCD patients. It was noted that these specific patients were in the age range of CD development and were already showing some signs of cognitive impairment. It was suggested by Lea et al. [23] that the FVIs sped up the process, resulting in a more eCD-like timeline of disease (ie rapid disease progression leading to death in a few months post initial disease signs), possibly explaining their diagnosis as eCD instead of CD patients. The eCD samples contained both homozygote and heterozygote individuals, suggesting that KashA dominates over KashC phenotype. It appears that KashA homozygotes exhibit more severe symptoms than the heterozygotes (Data not shown); however, this observation requires further investigation to be validated. Almost all of the healthy FVI wookie samples were KashC+, further supporting KashA as a potential causal factor for eCD in wookiees exposed to FVI. The small group of healthy FVI KashA+ wookiees was found, following an investigation by the Jedi order FDA (Jo-FDA), to be injected with a defective viral vector batch that did not include the mdDNA Force-enabling mutations. This provides an explanation why the KashA+ wookiees did not develop eCD post-FVI. Further investigation revealed incomplete penetrance of the KashA isoform in the familial, late-onset CD population. Compared to the general population where the KashA isoform was identified in approximately one fifth of the general population, mainly in the heterozygous form, a relatively large number of KashA+ individuals develop CD; this suggests that KashA in somehow genetically linked to the disease, increasing the risk of developing CD.
In this work, two consistent biomarkers of eCD, CSF Aβ and PET-PiB MCBP, were used. CSF Aβ data revealed a significant KashA x FVI interaction, with KashA+ individuals showing lowered levels of CSF Aβ when undergoing FVI. PET-PiB data mirrored this result, showing a significant KashA x FVI interaction, such that KashA+ wookiees with FVI having highest MCBP levels. Some confounds, such as socioeconomic status, subtle cohort bias (i.e. bias in those willing to participate in the study) may not have been addressed, and may have influenced the results. Furthermore, while this study provides a strong correlation between FVI use and eCD development, its design does not allow for causal inferences.
Both CSF Aβ and PET-PiB MCBP data in wookiees mirrors what is observed in the human condition. Interestingly, like in AD, eCD is more commonly observed in females. [24] Significantly lower levels of CSF Aβ in KashA x FVI females was found when compared to males. This parallel is useful in further characterizing eCD, as it is relatively uncharacterized compared to the human AD. Some differences were observed, however. In particular, in AD, there is a significant effect of age on CSF Aβ and PET-PiB MCBP, with lowered CSF Aβ and heightened PET-PiB MCBP with age. This was not observed in eCD. This could be due to the likelihood of eCD being highly influenced by FVI status, regardless of age. Humans are more prone to learned Force development, and a FVI has not been necessary for human Force acquisition.
Post-mortem data indicated the presence of amyloid plaques and NFTs in the cortices of CD-afflicted wookiees. In particular, wookiees with a history of FVI use exhibited the highest amounts of amyloid plaques and NFTs. Unfortunately, data on KashA status was not included, as blood samples were unavailable. Future studies should establish the relationship between KashA status and amyloid plaque/NFT burden in post-mortem samples. As with the CSF Aβ and PET-PiB MCBP data, similar post-mortem trends were observed when compared to AD.
In the human condition, while amyloid plaques are important hallmarks in determining AD status, the actual clinical symptoms are correlated with neurodegeneration, not amyloid deposition. [25] Furthermore, amyloid deposition alone cannot produce clinical symptoms. [25] This is likely to be true in eCD as well. For this reason, future studies should further characterize the patterns of neuronal loss on eCD, which may lead to the development of therapies to combat clinical symptoms of eCD.
In addition, the function of the Kashyk lipoprotein in wookiees is not yet fully known. In humans, this apolipoprotein serves many functions. Importantly, apolipoprotein clears Aβ aggregates, thereby preventing amyloid plaque formation. [25] APOE2 and 3 are termed “good APOE” as they can successfully clear Aβ. [25] APOE4, on the other hand, has a weaker ability to successfully clear Aβ aggregates, thereby facilitating the formation of Aβ aggregates, and leading to the formation of fibrillar Aβ, also called amyloid plaques. [25] It is likely that Kashyk lipoprotein plays a similar function, but future studies need to establish its role in wookiees.
Conclusions
The main objective of this work was to examine a potential genetic basis for discrepancies of eCD diagnosis following use of Force-vector injections. Based on the data presented here, a likely explanation for this phenomenon is the presence or absence of the KashA isoform of the Kashyk gene. The results of this research clearly reveals the importance of mandating a required screen for KashA prior to FVI administration to ensure no development of eCD. Based on the findings of this research prophylactic genetic screening will prevent the current rise in eCD, thus reducing the impact of this disease on the wookiee population.
Acknowledgments
The authors would like to thank the Kashyyykian Institute of Health Research, as well as the Alderaan Institute of Health, for providing funding for this project. Furthermore, the authors would like to acknowledge the Wookie organ database for providing post-mortem brain samples.
References
[1] Russell G, Rocheleau G. Genetic basis for Force-sensitivity in Wookiees. (2013) Annals of Praetachoral Mechanics 1:21-28.
[2] Solo H, Lea P. Targeted AAV injections of mdDNA in adult Wookiees. (2013) Science 23:45-55.
[3] Jabba TH. Modified Force-enabling stem cell transplantation in adult male Wookiees. (2013) StemCell Science 459:4995-5050.
[4] Yoda, Obi One K, Darth TMS midi-clorian retrovirus enables stable and sustained Force use in adult Wookiees. (2013) Nature 503:450-480.
[5] Dooku C, Fett B, Fett J. Central nervous system pathology of early-onset Chewheimer’s disease. (13 ABY) Journal of Chewheimer’s disease 24(5):345-361.
[6] C-3PO and R2D2. Progressive dementia in wookiees: a case study. (15 ABY) Annals of Neurology 33(4):134-150.
[7] Kenobi OW. An epidemiologic study on the rising prevalence of early-onset Chewheimer’s disease. (20 ABY) Journal of Neuroscience 43(1):620-635.
[8] Calrissian L, Amidala P, Bane C, Grievous G. A survey on prevalence of Force-vector injections in the Kashyyykian population. (20 ABY) Journal of Wookiee epidemiology 44(54):1990-2001.
[9] Calrissian L. Proapoptotic gene activity is enhanced in an Anooba Tatooinis model of early-onset Chewheimer’s disease. (21 ABY) Journal of Neuroscience 44(4):340-353.
[10] Head D, Bugg JM, Goate AM, Fana AM, Mintun MA, Benzinger T, Holtzman DM, Morris JC. Exercise engagement as a moderator of APOE effects on amyloid deposition. (2012) Archives of neurology 69(5):636-643.
[11] Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, Lopresti BJ, Ziolko S, Bi W, Paljug WR, Debnath ML, Hope CE, Isanski BA, Hamilton RL, DeKosky ST. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. (2008) Brain 131:1630-1645.
[12] Skywalker L. Evidence for amyloid plaque deposition and hyperphosphorylated tau in post-mortem wookiee brains. (15 ABY) Nature Neuroscience 80(11):960-978.
[13] The Hutt J. Comparing Blackwing virus pathogenesis and disease course in humans and wookiees. (14 ABY) Journal of Virology 87(3): 435-442.
[14] Maul D, Oppress S. The pathology of human asthma correlates significantly with wookiee asthma. (18 ABY) Journal of Respiratory Physiology 73(12):769-780.
[15] Solo H. Oxidative damage in early-onset Chewheimer’s disease involves activation of Bcl. (12 ABY) Journal of Chewheimer’s disease 22(4):981-1000.
[16] Iranpur VM, Esmailizadeh AK, Rapid Extraction of High Quality DNA from Whole Blood Stored at 4oC for long period. (2010) Protocol Online. URL: http://www.protocol-online.org/prot/Protocols/Rapid-Extraction-of-High-Quality-DNA-from-Whole-Blood-Stored-at-4-C-for-Long-Period-4175.html
[17] Ingelsson M, Shin Y, Irizarry MC, Hyman BT, Lilius L, Forsell C, Graff C. Genotyping of Apolipoprotein E: Comparative Evaluation of Different Protocols. (2003) Current protocols in Humans Genetics Unit 9.14
[18] Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid
imaging load and cerebrospinal fluid Abeta42 in humans. (2006) Ann Neurol 59:512–519.
[19] Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. (2006) Neurology. 67:446–452.
[20] Corbett BA, Simon D. Adolescence, Stress and Kashyk in Wootism Spectrum Disorders. (2000) Wootism Res. 23:23-30.
[21] Varvara P, Varuzza P and Vicari S. Executive functions in developmental Wookielexia. (2003) Front. Wookie Neurosci. 299:69948-70001.
[22] Young KP, Chang DJ, Reardon CA, Nucleotide sequence and structure of the human apolipoprotein E gene. (1985) Proc. Natl. Acad. Sci. 82(10):3445-3449.
[23] Lea P, Solo H. The effects of Force vector injections on Chewheimer`s disease patients. (2014) Journal of Chewheimer’s disease 27(2):95-101.
[24] Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. (2011) Neurobiology of Aging 32(4):604-613.
[25] Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CRJ, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. (2011) Alzheimer’s & Dementia 7:280-292.