HOW TO CATCH A CANCER
(August 2003)
Cancer: A Familiar Name, but What Does it Mean?
Cancer refers to the hyperproliferation of cells that have lost the ability to be controlled by normal cell signals. Cancer cells have the ability to proliferate independent of their environment and are capable of metastasizing, or colonizing other tissues in the body.
There are three basic characteristics of early cancer cells. The first is that they have lost the ability to undergo apoptosis, or programmed cell death. Cells that have suffered irreparable DNA damage activate specific proteases and nucleases that destroy the proteins and DNA of the cell, thereby effectively limiting the spread of potentially deleterious mutations. Cancer cells have often obtained mutations in genes involved in regulating this pathway.
Secondly, cancer cells have lost the ability to stop dividing. Normal cells require extracellular signals, such as growth factors, in order to activate pro-growth pathways. This need for extracellular stimulation is one means of regulating cell growth that cancer cells manage to bypass. This means that cancer cells proceed through the cell cycle and continue to divide indefinitely.
Thirdly, cancer cells often have abnormal telomerase activity. Telomeres are the specialized regions on the ends of chromosomes that form t-loops – these protect the ends and distinguish double-stranded DNA breaks from normal ends. Telomerase is an enzyme that acts to lengthen the telomeres on aging chromosomes in certain cell types. A portion of the telomere is lost during each DNA replication. When telomeres are shortened to the point that they cannot form t-loops, cells undergo senesence, where the cell remains in an undividing state, or apoptosis. Cancer cells, which are continually dividing, often express telomerase or have increased telomerase activity in order to circumvent the telomere problem. These three basic characteristics are the premise for malignancy and enable the accumulation of more genetic and chromosomal abnormalities, which further lead to increased malignant and metastatic phenotypes.
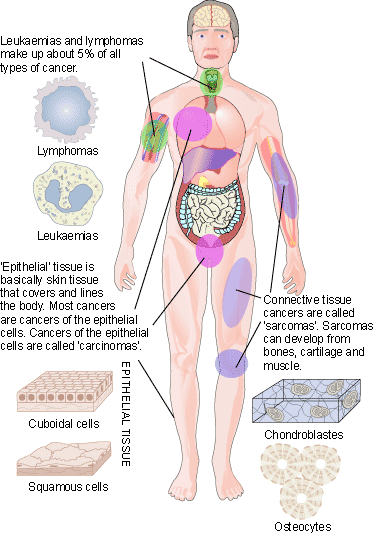
There are several different kinds of cancer but they all essentially fall into three basic categories: carcinomas, sarcomas and leukemias/lymphomas (see Figure 1). Both carcinomas and sarcomas are solid tumours, or tumours consisting of a dense collection of cancer cells that has its own blood supply. Carcinomas are solid malignant lesions originating in the epithelial tissue. These are often found in glands or the lining of various organs. Examples of carcinomas would be breast cancer and prostate cancer, and occur most frequently in older patients. Sarcomas are solid tumours of the connective tissue, such as bone and muscle. There is a higher incidence of sarcoma in younger patients than in older ones. Finally, leukemia and lymphoma are malignancies originating in immune or haematopoeitic cells and can be found in patients representing a wide age range. These cancers are commonly referred to as disseminated cancers as they do not form masses of cells, but instead are found throughout the vascular system.
How Does Cancer Arise?
Cancer arises most commonly in older patients but can occur at any age. Different cancers are more prevalent in certain age groups and are rarely found in people outside that age range. An example would be prostate cancer, where it is very uncommon for a man under the age of 40 to be diagnosed with the disease. It makes sense that cancer occurs predominantly in the elderly population, as cancer is the result of accumulated genetic mutations. As an individual ages they are more likely to have been exposed to chemicals, radiation and other events that cause DNA damage. As a result, these individuals are more likely to have mutations in genes allowing for cancerous behaviors.
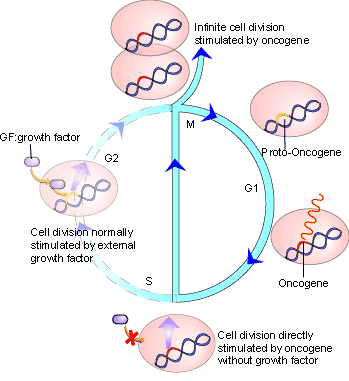
As mentioned above, characteristics like the ability to evade apoptosis, unregulated cell division and increased telomerase activity are all the results of genetic mutations that can lead to cancer. These mutations commonly occur in what are called proto-oncogenes and tumour suppressor genes. Proto-oncogenes are genes coding for proteins involved in cell cycle progression and growth signalling; when mutated these genes are called oncogenes (see Figure 2). Oncogenes are autosomal dominant, meaning that only one mutated allele is necessary for cancerous behavior to develop. Several oncogenes have been identified in many types of cancers, including myc, the bcl family of genes and ras[4].
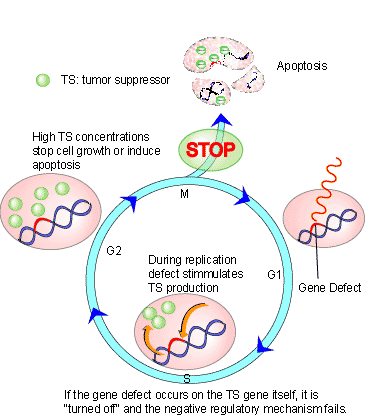
In contrast, tumour suppressor genes are often involved in regulating apoptosis (see Figure 3). Cells that have sustained damage to their DNA apoptose under the control of tumour suppressors; when both alleles are mutated to be non-functional cells fail to enter the apoptotic pathway. These cells continue to divide and are not subjected to further DNA repair attempts, resulting in increasing genomic instability and further mutations. These mutations often occur in other oncogenes and tumour suppressor genes, conferring more cancerous behaviors. If either of these kinds of mutations exists or occurs in someone then they are at a higher risk of developing cancer [4].
It is important to note that cancer results from the clonal expansion of one progenitor cell, or “mother” cell (the one that first obtained a mutation allowing for a “cancerous” phenotype). This means that all cells in a tumour should in fact be genetically identical to the parent cell and exhibit the same mutations, however, because mutations occur at a much higher rate in tumour cells, daughter cells may be more tumourogenic than the parent [4]. All that is required for cancer to develop is one cell among millions that is capable of developing independent of regulatory signals.
How to Find a Cancer Cell in a Haystack
If someone has cancer, how do we know? Early detection is crucial in the chance for cure but how can we detect a cancer before it’s too late? After all, tumours behave as exponential entities, where clinical detection can happen at about 1 billion cells and death can occur three doubling times later at 1 trillion cells. To complicate matters, a cancer is rarely detectable by physical or radiographic means at a mass lower than a billion cells (about 1 cm in diameter).
Traditional methods for determining tumour growth exist, and although somewhat simple are still effective. Three commonly occurring cancers (breast, testicular and prostate) are often detected early in development simply by palpation and touch. For either breast or testicular cancer, examining the area with the fingers in search of abnormal lumps is an effective method of detection. For prostate cancer, a physician performs a digital rectal exam and feels for enlargement of the prostate [3,4].
Melanoma, or skin cancer can be determined visually by looking for abnormal skin colouration or a large and discoloured mole. Of course, these methods are somewhat crude and signify only the possibility of cancer and cannot be used for such cancers that exist within the body, like lung cancer or colon cancer. Other symptoms exist which, when present, could indicate the presence of cancer. These include changes in bowel or bladder habits, blood in the urine, stool or sputum, a sore that doesn’t heal, indigestion or difficulty swallowing and others.
More direct methods, such as molecular and biological techniques are also used in conjunction with biopsy and microscopic/pathological examination [3]. Often physicians rely on detection of cancer using chemical tests because the cancerous cell has undergone molecular changes that differentiate it from normal cells. For example, in prostate cancer the prostate produces high levels of PSA (prostate specific androgen) which can be detected in the blood or urine using the PSA test [1,3,4]. Interestingly, following the emergence of the PSA test the incidence of prostate cancer rose dramatically but the number of early detections had increased and therefore mortality is lower.
X-ray imaging is another detection method that can be used if the tumour is slightly larger in size. Tumours appear as a dense mass in X-rays, although this does not indicate whether the tumour is benign or malignant. Further analysis using other tests, such as a PET scan, is required to determine if the tumour is a cancerous entity. A mammogram is simply an X-ray of the breast used as a preventative/early detection mechanism [2,4].
The blood test AMAS (anti-malignin antibody screen) is a general test for detecting any kind of cancer. Its accuracy is reported to be greater than 95% and with repeated testing increases to over 99% with a less than 1% false positive/negative. This blood test works on the premise that tumours secrete the antigen malignin, which is not secreted by normal cells. By using an antibody against malignin, low levels of the protein can be detected with molecular techniques such as Western blot analysis. One limitation of this test is that it is only capable of detecting tumours during early stages of development; as a tumour matures and mutates malignin is no longer secreted. This test can be used long before any other conventional medical test, and is also a safer alternative to other techniques that rely on the use of radioactivity or other harmful substances.
The BTA (biological terraine assessment) is a computerized test which examines the urine, blood and saliva for the numbers of electrons present, pH balance and other minerals present in these fluids. Depending on the balance of factors tested, it can be determined whether cancer is likely present or not [3].
There are a number of marker tests in use which look for specific molecular markers that signify the presence of malignant cells. These types of tests are vital not only for early detection, but also for monitoring the progression of a cancer. As a tumour grows and changes it exhibits different markers — by determining which marker a tumour is displaying one may determine whether the tumour is in its early or late stages of development. One such marker is alpha-fetal protein levels, which are often raised in cancer patients and pregnant women [3]. The levels of this protein are elevated in cancer patients because tumours tend to revert to fetal characteristics, which is one of the ways they avoid detection by the host immune system as well. It is in a sense a de-differentiation of the cell.
In addition to marker tests there are other tests that focus on the morphology of tissues or cells in an attempt to identify abnormal growth. These tests include endoscopic ultrasounds, which examine internal organs to determine the general health of a tissue and whether or not there is a tumour present.
The lymphocyte size analysis is another diagnostic tool that works by examining the gross morphology of an individual’s lymphocyte population. By measuring the diameter and number of lymphocytes in the blood, this test can determine increases in swollen lymphocytes present, and subsequently, the likelihood of a tumour developing.
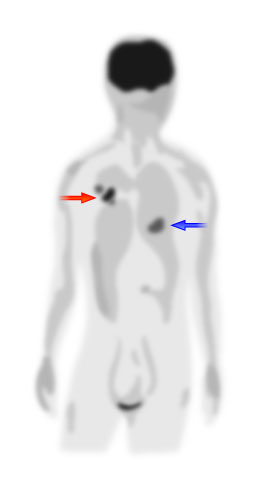
A PET (positron emission tomography) scan allows a doctor to detect possible malignant lesions by monitoring a patient’s metabolic activity [3]. By radioactively labelling glucose it can visualize glucose consumption throughout the body and identifies areas of high metabolism (see Figure 4) [1,2]. Since malignant tumours consist of dividing cells, these areas would have a high metabolic activity – any areas that exhibit abnormally high glucose consumption signify the likelihood of cancer. Unfortunately, this method is not as safe as a simple blood test due to its use of radioactivity to trace glucose molecules.
Other tests include using the thermal radiation a body emits to reconstruct thermal images of the body [3]. This technique identifies new blood vessels in the body, which is one of the steps in tumour development that precedes metastasis. This technique is limited though in that it cannot identify very small tumours (as these tumours do not yet require their own blood supply) and it cannot pinpoint the location of the tumour. Therefore, it must be used in conjunction with other methods of cancer detection.
Diagnosing Cancer Before It Strikes
With the development of molecular biology and genetics it is now possible to perform genetic screening that can identify individuals at high risk for developing cancer. A predisposition to develop certain cancers is heritable in the form of one mutated tumour suppressor allele. When an individual inherits one dysfunctional tumour suppressor gene, they only require one additional mutation in the remaining functional allele to completely lose the regulatory activity of that gene – this means a much higher chance of developing cancer than an individual with two functional copies [5].
Familial adenomatous polyposis (FAP) is one form of colorectal cancer that is known to have a genetic basis [6]. This form of cancer is passed to the next generation via inheritance of only one functional copy of the tumour suppressor APC. Individuals inheriting one mutated copy of APC only need to suffer a mutation in the other APC gene to have a complete loss of APC activity. APC normally decreases intracellular levels of β-catenin, a transcription factor that promotes the expression of growth and cell division genes; high levels of this transcription factor result in excessive cell division. Patients with FAP typically develop numerous tumours (or polyps) in their gastrointestinal tract, and a small number of these progress into malignant tumours [6].
By testing individuals with a family history of FAP it is possible to determine whether or not they carry a mutated allele of APC [6]. This has the double effect of identifying those at risk, and easing the unnecessary anxiety of those individuals that do not carry the mutation. Patients that test positive for the mutation are then closely monitored for the development of abnormalities in their gastrointestinal tract. Early signs of tumour formation can then be addressed using preventive prophylactic surgery, thereby preventing malignancy. Another reason for using genetic testing is to inform patients already diagnosed with the disease that theirs was the result of genetic or environmental factors, which may have consequences for the individual’s family.
Conclusion
Cancer is a serious health issue that affects each and every one of us in some way, whether it be directly or indirectly. Most cases of cancer occur in the elderly because aging has an impact on cellular integrity; however, many heritable childhood cancers exist as well. Improving therapeutic outcome and decreasing the number of deaths caused by this complex disease require its detection at an early stage — before a tumour begins to metastasize. There exist a number of tests of all different kinds ranging from blood tests to radiography to marker tests to thermal imaging, all enabling detection of malignancy. With the advent of genetic screening, it is now possible to identify people at high risk for developing cancer – allowing more effective treatment at earlier stages of development.
References
1. Sass J.O., Pascoe-Gonzales S., Lehnert W. (2002). Screening in clinical trials. The Lancet. 360: 952.
2. Introduction to detecting cancer
3. Laboratory Tests that Detect Cancer
4. Tannock I.F., Hill R.P. The Basic Science of Oncology, 3rd ed. New York: McGraw Hill Companies Inc., 1998.
5. Hahn M., Saeger H.D., Schackert H.K. (1999). Hereditary colorectal cancer: clinical consequences of predictive molecular testing. International Journal of Colorectal Disease. 14: 184-193.
6. Kinzler K.W., Vogelstein B. (1996). Lessons from hereditary colorectal cancer. Cell. 87: 159-170.
Additional Reading
1. Weinberg R.A. One Renegade Cell: How Cancer Begins. New York: Basic Science, 1998.
=> Very easy read explaining cancer progression.
2. Hanahan D., Weinberg R.A. (2000). The hallmarks of cancer. Cell. 100(1): 57-70.
=> An excellent review on the genetic basis of cancer.
(Art by Fan Sozzi)