FORCING THE WOOKIEE. HOW TO BE BOLD BEING BALD.
Annals of Praetachoral Mechanics (2014). Vol 1. pp79-87 download pdf
ABSTRACT
The lack of scientific findings in the biological relationship with the Force has limited the therapies available against Force hypersensitivity to psychological therapy. Our lab has unearthed the first link between the Force-enabling midi-chlorians and a regulated biological process, hair growth. Alopecia areata (AA) is a hair condition depicted by hair loss with varying degrees of severity. A recent study has revealed a strong correlation of AA with enhanced connection to the Force. Exome sequencing has revealed two hot spot mutations in the transmembrane protein “so much hair” hormone receptor (smhHR) and is implicated in more than 80% of Wookiee AA cases. Furthermore, we demonstrated that the activity levels of midi-chlorians in the Wookie stem cells residing in hair follicles are decreased due to the activation of smhHR by binding to its natural ligand, smhH. smhHR activation is weakened in the hot spot mutants due to a conformational change that decreases the binding affinity of smhHR to smhH. The resulting increase in force-sensitivity can be restored to normal Wookiee levels by the introduction of potent agonists or plasmids expressing wild-type smhH. Thus, our study has discovered potential therapeutics against AA as well as Force-sensitivity suppressants.
Keywords: Alopecia areata, Wookiee, midi-chlorians, smhH, smhHR
Introduction
Force Control Dysfunction (FCD) is a condition characterized by hyperactivity of midi-chlorians, the life-forms responsible for an organism’s connection to the Force [1,2]. This results in a phenomenon termed Force Hypersensitivity, wherein an organism is able to strongly manifest the Force, but is unable to control its energy [1,2]. In particular, Carlisan et al. have studied human Jedi in training with this condition, and found the common symptoms to be fear, anger, and in the most advanced form, hate and suffering [2]. Although only 10% of young padawans are affected by FCD, it is known that all Dark Jedi (corrupted Jedi that have switched to the Dark Side) had this condition [1,2]. This indicates urgency for FCD therapies. The difficulty in finding therapeutics exists in the fact that the causes for this condition are not entirely known and is complicated by the restrictions set on Force-related studies, especially in humans. Recent work in our lab has found that Wookiees with the hair growth disorder, alopecia areata, present with a symptom of Force-sensitivity [3]. This is the first indication of a correlation between midi-chlorian activity and another regulated biological process in any species [3].
Wookiees are generally impaired in their connection to the Force [1,3,4]. However, Wookiees with alopecia areata are exceptions to this generalization [1,3]. Alopecia areata (AA), is a hair growth disorder involving an underproduction of the ‘so much hair hormone’ (smhH), which is the peptide hormone responsible for hair growth in many species including Wookiees and humans [1,3]. Under normal Wookiee physiology, the constitutive expression of the FurRY gene signals the production of smhH in the pituitary gland [3,4].
SmhH then travels to the base of the hair follicles and acts on the follicular stem cells via the smhH receptor (smhHR) to trigger the Wookie Hair Yield (WHY) pathway [3,4]. Downstream signaling results in the allocation of energy and nutrients towards the process of hair growth in the follicular stem cell [3,4]. In Wookiees affected with AA, a defect in this signaling pathway results in patches of baldness in random areas of the body [3]. Although decreased available energy and nutrients for the WHY pathway has been implicated in this condition, the precise defect(s) in the hair growth mechanism responsible for AA had remained an enigma [3].
Apart from the apparent symptom of bald patches, these Wookiees with AA also exhibit Force-sensitivity and symptoms similar to FCD in humans [3,4]. For instance, these Wookiees have a greater ability to sense danger and are often more aggressive than their counterparts with normal hair growth [3,4]. These characteristics suggest a connection to the Force, contrary to the existing claims that Wookiees are not capable of connecting with the Force [3,4]. Indeed, recent work by Dahut et al. has analyzed the midi-chlorian count in Wookiees with AA, and has confirmed midi-chlorian activity comparable to that of pre-training human padawans [5].
As midi-chlorians are symbiotic life-forms requiring host-derived nutrients and energy to be active, we hypothesize that in dermal cells, the hair growth pathway competes with this nutritional demand. The goals of this study were to elucidate the mechanisms behind midi-chlorian activity and AA in Wookiees, and to determine the cross-talk between these two processes. Due to the close evolutionary ties of Wookiees to humans, the results of this study suggest potential therapeutic targets for FCD in humans, namely with the interaction of smhH and smhHR.
Methods
Cell culture
Constitutive Hair-Expression Wookiee-Y (CHEW-Y) stem cells and the immortal pituitary gland cell-line HSOLO were obtained from Wookbook Cell Database of Kashyyyk. A smhHR knockout was derived from CHEW-Y and was a kind gift from Dr. Orlov (undisclosed lab). Primary-cell samples were obtained as donations from bald patches of Wookiee AA patients at the Underground University Hospital of Kashyyyk by a biopsy procedure previously described by Derma et al [6]. Compensation was given to donors in the form of crossbows for each participant, sponsored by the Jedi Council (Coruscant). Primary cells were used within 5-8 passages. For all types of cells, Kitster’s Medium enriched with 10% fetal eopie serum was used [6,7].
Proteins
So much hair hormone, smhH, was produced in a baculovirus expression system as previously described [8]. Purification of smhH as well as smhHR and its mutant derivatives (described below) was done by His-tag purification. smhHR was removed from the membrane with dodecyl phosphocholine (DPC) [8]. Purified FurRY and WHY1 was purchased from SimiTechnologies (Polismassa, Galaxy3).
Force-sensitivity measurement by flow cytometry
Cells were harvested and pelleted at 300 x g for 5 minutes then washed three times with PBS by centrifugation. 1 x 106cells were fixated with 4% paraformaldehyde for 10 min at room temperature. 1:10000 sheep anti-midiC antibodies conjugated to biotin in permeabilization buffer/wash buffer (WyDetect Technologies) was added to cells and incubated for 30 minutes in the dark at room temperature. Cells were washed twice with permeabilization buffer/wash buffer. Flow Cytometry was performed with the Flow Reader (WyDetect Technologies). The fluorescence units were directly correlated with midi-chlorian activity [7]. For a negative antibody control, a sheep IgG isotype control antibody was used.
Exome sequencing
Cohort study data was accessed via StarBank. Specific-exome next generation sequencing was done at the Genome Institute, Underground University of Kashyyyk and in collaboration with Dr. Derma. A variety of platforms, capture reagents and sequencing platforms were employed, including MegaMorph and NimbleSeq arrays, Illuminati Array and Rocket sequencing, including custom resequencing and validation arrays [8,9].
Dose-response smhHR inhibition assay
To make the smhHR knockdown cells, siRNA directed towards the smhHR gene was designed and synthesized in our laboratory using the siRNA computational software ShutIT (WyDetect Technologies) [7]. The siRNA were transfected into the CHEW-Y cells using the DharmaFECT siRNA transfection kit (SimiTechnologies) according to the manufacturer’s instructions [8]. Knockdown effectiveness was validated by using siRNA to GAPDH as a positive control and non-targeting siRNA as a negative control. These knockdown models were compared to the smhHR knockout cells, along with CHEW-Y cells as wild-type control. All cells were grown to 70% confluency in 12 well plates, each type of cell in triplicate, and force-sensitivity measurement conducted on all samples.
Vector-plasmid reintroduction
FurRY, smhH, and smhHR genes were isolated by restriction digest from healthy Wookiee scalp cells and inserted into the lentiviral vector pLVX-IRES-ZsGreen1. The plasmid was transformed into the cell-cultures by lentivirus infection. The effectiveness of the transformation was verified by conducting agarose gel-electrophoresis on the cell-lysates to confirm the presence of the vectors (data not shown). Each gene reintroduction was performed in triplicates. Force-sensitivity measure-ments were conducted on all samples.
Site-directed mutagenesis
Alanine substitutions in full-length smhHR at Thr-304, Thr-369 and both Thr (denoted as smhHR-304, smhHR-369 and smhHR-304/369, respectively) were performed by PCR. We used site-directed mutagenesis by primer extension (WyClone Laboratories) [8,10]. 5x-Histidines were added to the N-terminal of the protein for purification by nickel-chelating column. Full length
amplicon primers used (5’ → 3’): forward, ATGCATCACCATCACCATGCCACTACGCCCAGC; and reverse, TGCAGTGAGCCAAGATTGTGCC. Thr-304 mutant required two additional primers in reaction: forward A, GAGACTTTTTCATGCGCCTGGACAGATGGGGTTC-; and reverse B, GAACCCCATCTGTCCAGGCGCATGAAAAAGTCTC. Thr-369 included forward C, CTGTAGTCGCTAGGCCGCCCCTAGTCAGGTTTCG; and reverse D, CGAAACCTGACTAGGGGGCAGCTAGCGACTACAG. A double substitution was made by using the Thr-304 mutant as a template with the Thr-369 primer mix. The resulting mutations (underlined) were confirmed by restriction digestion and nucleotide sequencing.
Binding assay
Radiolabelling of smhH with I125 was done as previously described [7]. smhHR was incorporated into unilamellar phospholipid vesicles composed of 20% phosphatidylserine and 80% phosphatidylcholine to maintain proper orientation of the transmembrane protein. Incorporated smhHR was subjected to increasing concentrations of I125-smhH as per BindIt protocol (WyDetect Technologies) [7]. Analysis of binding properties was automated through gamma counting by BindIt Analyser (WyDetect Technologies).
smhHR functional assay
A chromogenic substrate, S-90210 (obtained from WyDetect Technologies), is incubated with 500 nM smhH to ensure saturation of smhHR. The cell-permeable S-90210 is then cleaved by the WHY tyrosine kinase to release a colored compound that can be detected with a spectrophotometer. The units are displayed as OD/min.
Native gel mobility shift assays
8% native polyacrylamide gels were prepared by mixing 8% of 30:1 acrylamide to bis-acrylamide, Tris-HCl buffer, and APS and TEMED as the polymerization catalysts. The proteins were run in the gels for 45 minutes at 175 volts. A Western blot transfer was conducted on the gels usind PVDF membranes. Anti-smhHR antibodies purchased from SimiTechnologies were used for detection of bands.
Dose-response force-sensitivity assay
HSOLO and AA primary cell-lines were co-cultured using transwells, with the primary cells on the plate surface and the HSOLO cells on the inserts. Both cell-lines were grown to 80% confluency in complete Kitster’s Medium before addition of the respective concentrations of the purified hair growth pathway proteins. The proteins were incubated with the cells for 4 hours before force-sensitivity measurements were taken. Each sample was conducted in triplicates.
Statistical methods
A two-tailed student’s t test was used to determine P-values for treatment conditions compared to their respective control. P < 0.05 was used to determine significance.
Results
Wookiees with alopecia areata have hot spot mutations in their smhHR gene.
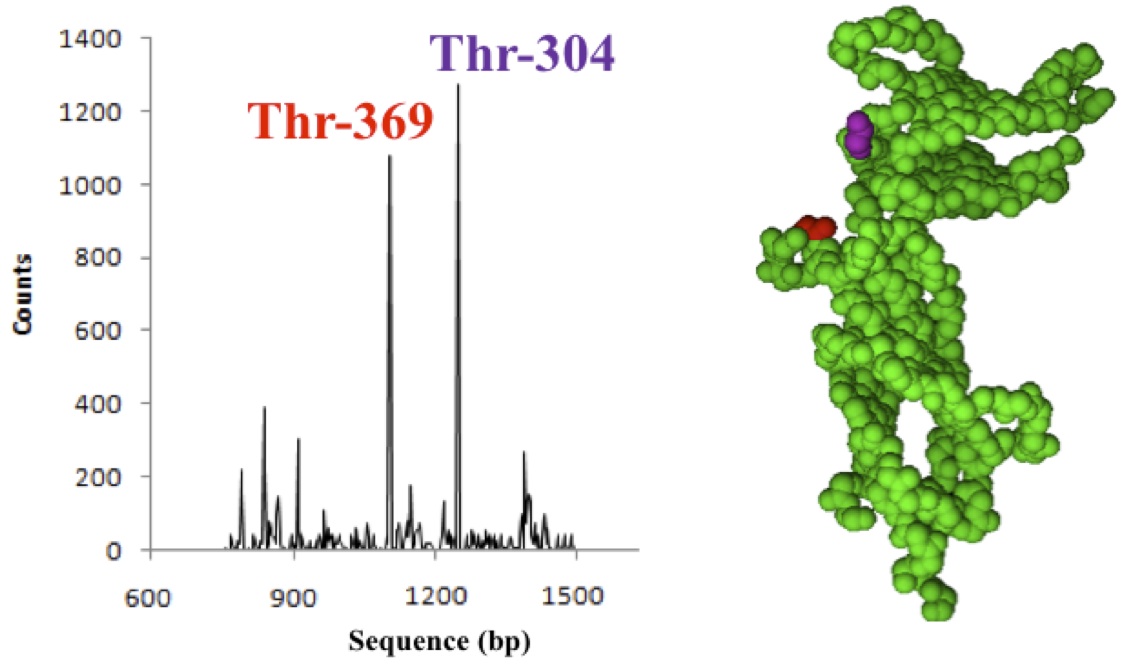
We performed a meta-analysis of 13 exome studies on a total of 1465 Wookiees experiencing moderate to severe forms of AA by exome sequencing. 1277 (87.2%) of the Wookiees had genetic alterations in the smhHR gene. From this finding, we identified two hotspot mutations where single nucleotide polymorphisms occurred (Fig 1). Of these 1277, 1082 (84.7%) of the Wookiees had a missense mutation at position 1107 in the ORF of smhHR that encodes for threonine, Thr-304. 1240 (97.1%) have a similar missense mutation at position 912 that also encodes for a threonine residue (Thr-369). However, only 795 of individuals carrying smhHR mutations (62.3%) suffered from severe AA. This suggests that other factors contribute to the condition.
Nonetheless, the average midi-chlorian activity in Wookies (3250 fU +/- 181 fU), experiencing moderate to severe forms of alopecia was greater than Wookies with mild or no alopecia (data not shown). Ergo, smhHR is a possible explanatory variable in the relationship between alopecia and force-sensitivity.
Activation of smhHR results in increased force-sensitivity.
To further validate and elucidate the interaction of midi-chlorian activation with the smhHR activation stage, we conducted a dose-response assay testing differing levels of smhHR inhibition in CHEW-Y cells. We used a knockout model of smhHR (smhHR-/-) to represent a total inhibition of receptor activation. For a ‘moderate inhibition’ model, we knocked down smhHR expression using siRNA directed towards the smhHR gene.
Figure 1. (Click to Enlarge) Hot spot single nucleotide polymorphic mutations found in the smhHR gene. The affected amino acids are highlighted in the smhHR backbone structure (right): Thr-304 (red) and Thr-369 (magenta). Detected mutations were only counted when above normal tissue mutational background (P < 0.05).
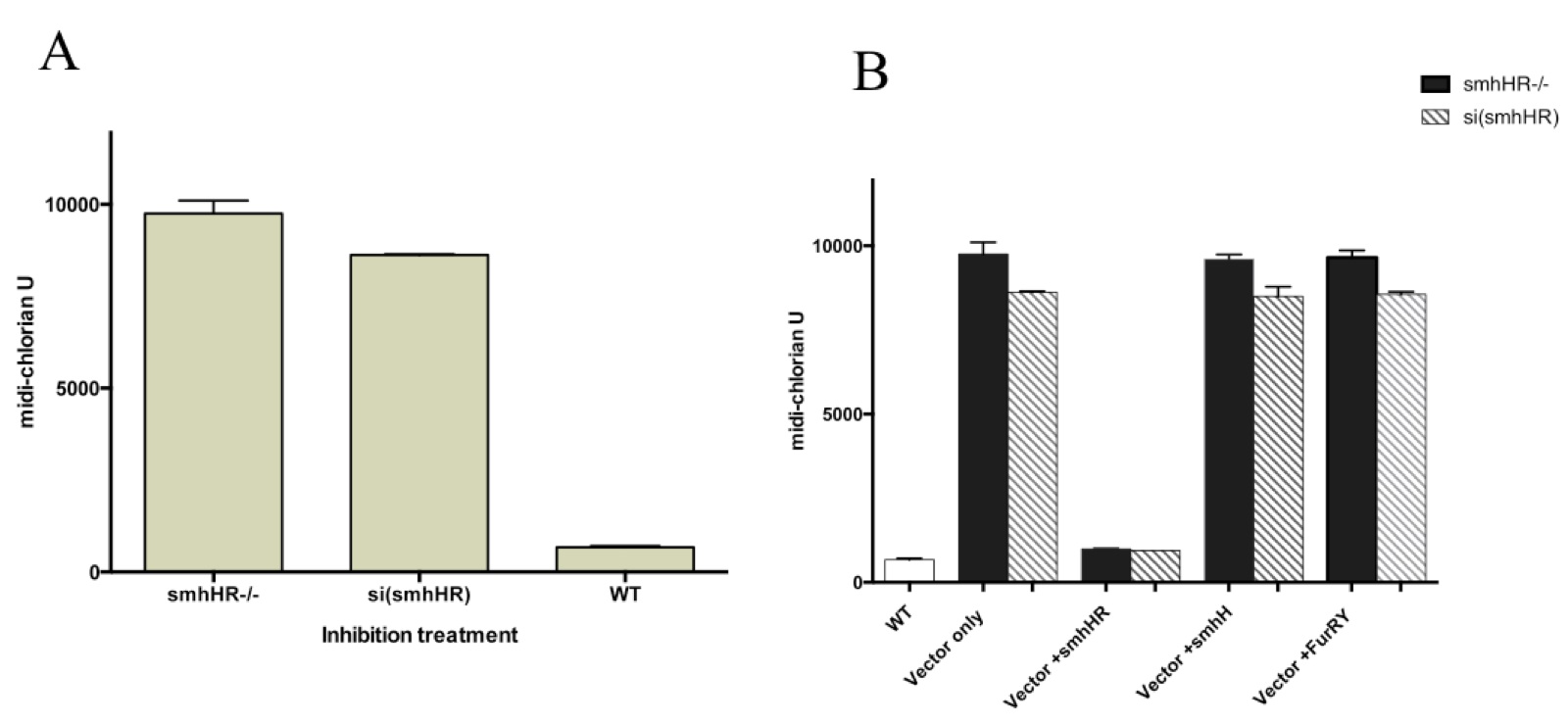
The results show a dose-dependent effect, with the highest level of midi-chlorian activity observed in the knockout model, a slightly decreased level in the knockdown model, and finally, the lowest level observed with the wild type CHEW-Y cells (Fig 2A). This was supported by the decrease in midi-chlorian count to almost baseline levels after re-introduction of a functional smhHR gene via a vector plasmid to both the knockout and knockdown models (Fig 2B).
Figure 2.(Click to Enlarge) Midi-chlorian activity pathway is influenced by the hair growth pathway via the smhHR. (A) smhHR gene expression inhibition in CHEW-Y cells by gene knockout or gene knockdown with siRNA resulted in an increase in force-sensitivity, as measured by the midi-chlorian count. Both are compared to wild-type CHEW-Y cells without gene inhibitors, and were both found to be statistically significant (p>0.01). (B) Introduction of functional genes in the upstream pathway of hair growth, added to both knockout and knockdown smhHR. Only vector induction of the smhHR restored the midi-chlorian levels to wild-type levels (significantly reduced from the empty vector controls, p<0.01).
On the other hand, the baseline midi-chlorian level was not restored upon introduction of vector plasmids containing either functional smhH or FurRY genes, indicating that these components in the hair growth pathway are not crucial in the interaction with midi-chlorian activation. Furthermore, this points towards smhHR as a potential player in the pathogenesis of AA.
smhHR mutations decreases the binding affinity to its natural ligand, smhH, but does not affect smhHR signaling ability
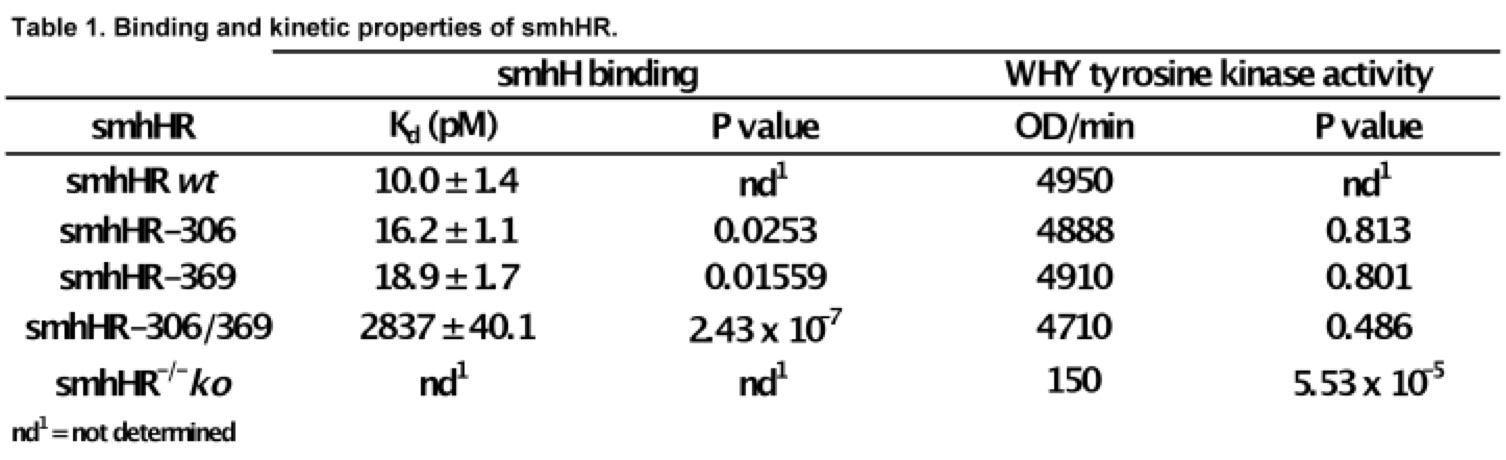
From sequence analysis, the two missense mutations occur in the hormone binding domain. Ergo, we expected a decrease in binding affinity to smhH. In order to assess the functional consequence of the mutations, we replaced the Thr with Ala. Our binding assay demonstrated a significant increase in the dissociation constant (Kd) of the single smhHR mutants to smhH (Table 1). Yet, there appears to be a large synergistic decrease change in Kd when both mutations are present (Table 1). In terms of activating the WHY pathway, none of the Ala-substituted mutants had functional defects in activating the signaling WHY tyrosine kinase (Table 1). Hence, the missense mutation at Thr-304 and Thr-369 only affects the recognition and binding of smhH.
Midi-chlorian activity is increased in mutated smhHR (AA) primary cell-lines
To confirm the relevance of our in vitro findings to a biological disease model, we conducted midi-chlorian activity assays using primary cell-lines from 12 different age-matched Wookiee AA patients. The cell-lines were categorized into either ‘severe AA’, ‘moderate AA’, or ‘mild AA’ by a previously described phenotypic ranking. Since it is believed that several genes are involved in regulation of hair growth, we aimed to characterize only the smhHR protein profile in each patient cell-line.
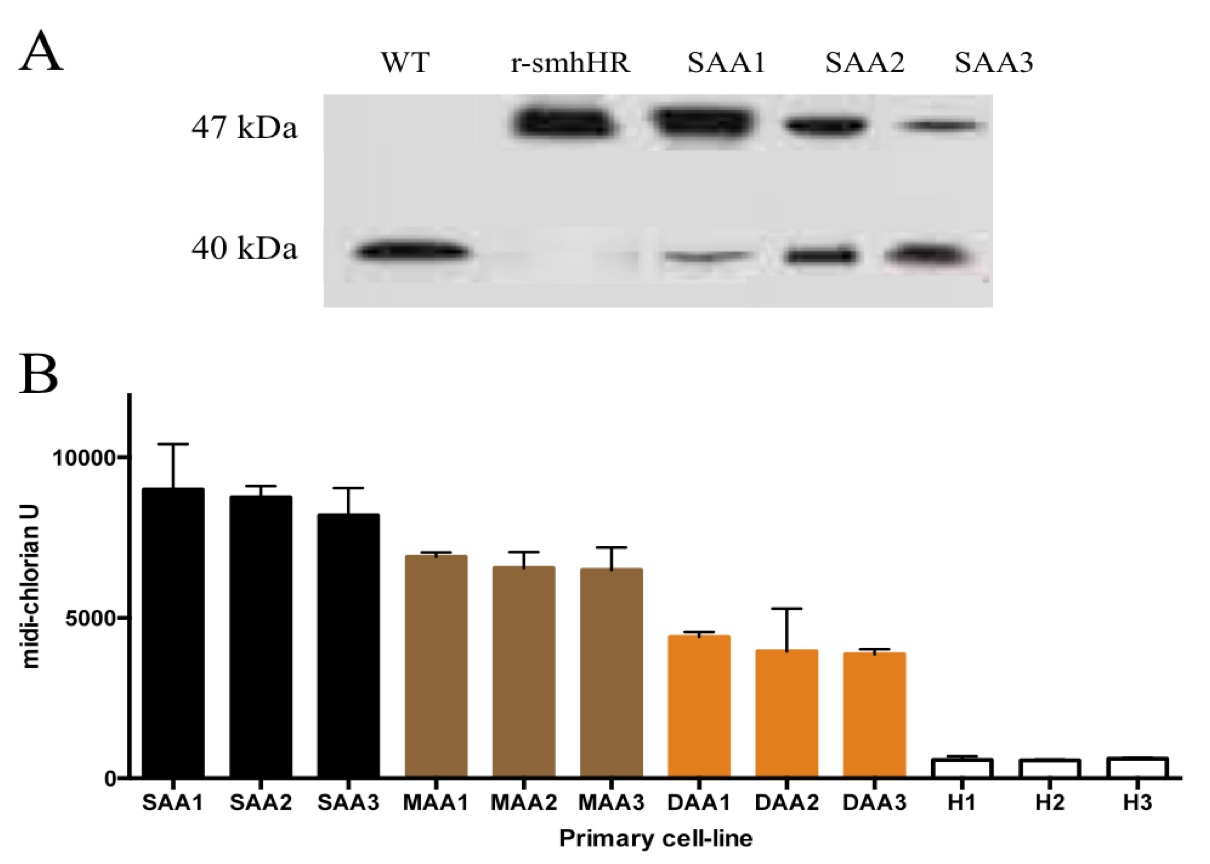
From the exome sequencing studies, we were able to make recombinant smhHR mutated proteins to be used as control for the subsequent studies. Native gel mobility shift assays confirmed a conformational mutation in the recombinant smhHR, demonstrated by the shift of the protein band upwards as compared to the smhHR from CHEW-Y cells (Fig 3A). The presence of a band in the same location for the primary AA cell-lines further strengthens the association of this mutation in AA. Not only is this band present in all the primary AA cell-lines, but the intensity of the bands varies according to each AA category. From the mild to severe designation, there is a trend towards a decreasing level of wild-type smhHR, and a simultaneously increasing level of the mutated smhHR band (Fig 3A).
Concurrent with the native gel assays, the midi-chlorian levels of the primary AA and healthy cells were measured after being grown to a common baseline cell number. In line with our previous findings, the trend of increasing midi-chlorian levels follows the same trend of increasing amounts of mutated smhHR in the same group of cells (Fig 3B). This result validates the cross- activity via a smhHR-dependent mechanism in clinical talk between the hair growth pathway and midi-chlorian disease models.
Figure 3. (Click to Enlarge) Mutated smhHR in AA primary cells is associated with increased force-sensitivity. (A) Western blot of native polyacrylamide gel mobility shift assays. The 47 kDa bands represent the mutated smhHR, while the 40 kDa band is the wild-type smhHR. Purified wild-type smhHR and recombinant smhHR acted as positive controls for their respective strains. (B) Midi-chlorian count for every donor primary-cell line. ‘SAA(#)’ indicates ‘severe AA’, ‘MAA(#)’ indicates ‘moderate’ AA, ‘DAA(#)’ indicates ‘mild AA’, and ‘H(#)’ indicates healthy control samples. Cell-lines are arranged from highest to lowest midi-chlorian count, from left-to-right. The average for every category is statistically significant from each other (p<0.05).
Midi-chlorian activity can be restored to normal by addition of functional smhHR and partially restored by addition of functional smhH
Thus far, we have discovered that the smhHR plays a critical role in AA pathogenesis and consequently is the crucial component involved in the cross-talk between the hair growth and midi-chlorian activity pathways, as observed in AA Wookiee models. We then wanted to test whether the increased midi-chlorian activity caused by the mutant smhHR can be reversed by adding an excess of upstream hair growth pathway components to pituitary (HSOLO) and dermal cell co-cultures.
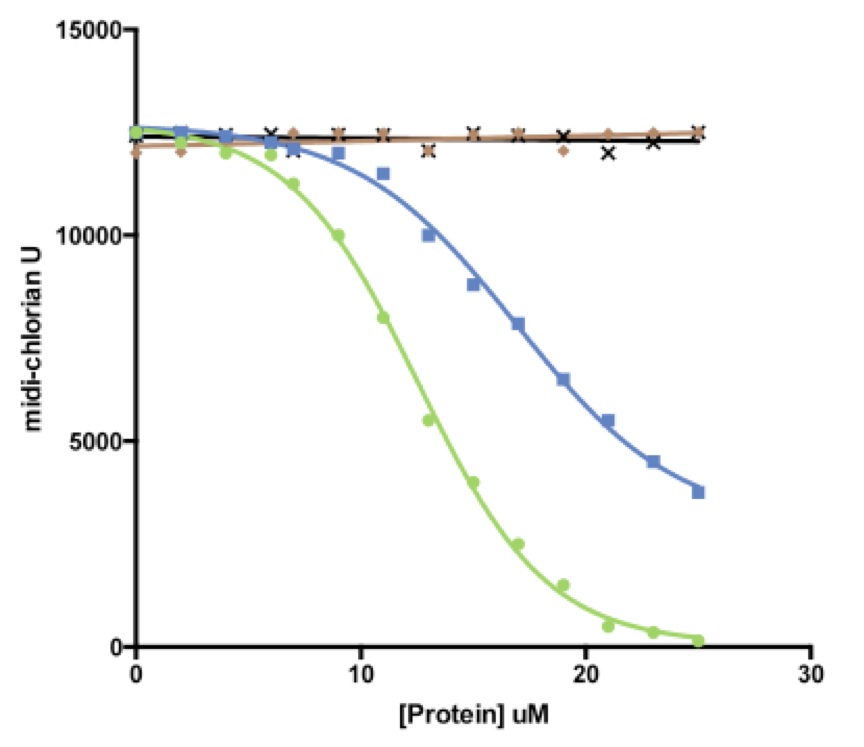
The primary cell-lines with the highest amount of midi-chlorian units found in the ‘severe AA’ patients was used as the dermal cells to test these restorative effects. Upon addition of increasing concentrations of FurRY protein, there was no significant change observed in the midi-chlorian activity from the baseline levels of the primary AA cell-line treated with a hair growth pathway upstream protein, as control. However, the addition of increasing smhH concentrations resulted in a slight shift to the right in the graph, with an IC50 that is 1.5 times of that in the smhHR addition curve (Fig 4). This finding is surprising since the introduction of the smhH gene via a vector had no significant effect on midi-chlorian levels (Fig 2C). Nevertheless, this result presented at least two compounds with potential therapeutic efficacy for suppressing hyperactivity of midi-chlorians.
Figure 4.(Click to Enlarge) Reintroduction of smhH and smhHR reverses force-sensitivity in AA primary cells. Increasing concentrations of hair growth upstream pathway components FurRY (u), smhH (n), and smhHR () effect on inhibition of force-sensitivity in ‘severe AA’ cells, measured in midi-chlorian units. WHY1 (✕) is the downstream protein control. IC50 for smhHR = 12uM, whereas IC50 for smhH = 22uM (extrapolated).
Discussion
In this study, we report for the first time, a mechanism by which AA contributes to force-sensitivity. We see that mutations that occur in the hormone binding region of smhHR are most common and affect the binding affinity of smhH most strongly when both are present. The rationale behind these hot spot mutations is not known. Even though almost all of the Wookiees contain either Thr-304 or Thr-369 mutations, only two-thirds of the Wookiees have severe AA. It is possible that there is a heterogenous population of these two single or double mutants which would describe the mutations as being somatic. To note, threonine is one of few amino acids that are O-glycosylated and the change in conformation may be due to this lost of glycosylation [1]. Perhaps both Thr are glycosylated to maintain the binding site for smhH and when one is removed, only partial collapse of the site occurs. This would support our observation of a smaller increase in Kd in single mutants than in double mutants. Even so, further studies on these hot spot mutations could shed light on the mutagenic events leading to AA.
In addition to being a key player in the pathogenesis of AA, these studies have shed some light on what were once an inexplicable phenomenon; the connection between Wookiee AA and their unusual sensitivity to the force. Indeed, the same defect in the same pathway involving the same mutation is implicated in midi-chlorian hyperactivity in Wookiees. Although we have narrowed down a point of cross-talk between midi-chlorians and the WHY pathway, the exact biochemical players have yet to be worked out. Still, this finding has greater implications for the well-being of the Galactic Empire. Young padawan who have Force-hypersensitivity pose a great danger to the overall balance of the Force. The biological mechanisms discovered in this study provide potential targets for therapy of FCD by suppressing Force-hypersensitivity.
A limitation of our study is that our exome-based approach only looked at dermal cells and did not take into account non-exon mutations (e.g. introns, promoters). Without financial limitations, whole-genome sequencing could be done. In particular, investigating the pituitary gland for genetic mutations may reveal mutations affecting smhH production that can help account for the smhHR-independent AA in Wookies.
The Wookiee genome has the greatest homology to humans than any other species in the known Galaxy [1]. Withal, smhHR shares 95% sequence homology with human growth hormone receptor [5]. Thus, the findings of this study are likely to be relevant to human pathologies as well. This provides more support for translating this study to human Jedi. However, it must be noted that humans comprise of only 70% of all Jedi levels. FCD in other species, especially those that are non-mammalian, require other disease models.
Acknowledgements
This work is supported by the Questionable Force Studies Society funding grant. We would also like to extend our thanks to David Ng of University of British Columbia, Dimension 2, Earth, Milky Way for providing us the opportunity to write this paper and for being the un-notified correspondent of this paper. We are grateful for our collaboration partner, Toll Derma, for doing all the exome sequencing. We would also like to acknowledge George Lufas for creating the redundant midi-chlorians as an attempt to quantify the Force.
Authorship
Contribution: B.L. and L.O. designed and dreamt of performing the research in a galaxy far, far away, analyzed data and wrote the paper in the likeness of Blood.
Conflict-of-interest: B.L. and L.O. have served as unpaid consultants for the Jedi Order.
Correspondence: David Ng, Michael Smith Laboratories, University of British Columbia, #301 – 2185 East Mall, Vancouver, BC V6T 1Z4, Canada; e-mail: db@mail.ubc.ca
References
1. Abon B. Hitchhiker’s guide to the galaxy. Best Guides. 2 ABY; 1(1):40-189.
2. Carlisan E, Joe P, HIUSHYYAT K, Juurgen B. Force hypersensitivity: A review of past literature. Best Rev. 3 BBY; 1:32-64.
3. Lin B, Ocariza L. Alopecia areata and Force-sensitivity in Wookies. Hairless. 20 ABY; 7: 12-40.
4. Guile B, Guile S. Wookiees of Kashyyyk: Mysteries to be solved. Kashyyyk Unlim. 10 ABY; 5: 55-555.
5. Dahut L, Dahot O, Dahet V, Dahat E. The Force in balding Wookies. Force Me To Read. 10 BBY; 1:10-6048.
6. Derma T, Ologis T, Are C, Ool M. Biopsy of alopecia areata patch samples. Biopsy 101. 103 BBY; 2(1):30-36.
7. WyDetect C. WyDetect manually when you can do it automatically. Kashyyyk Unlim. 1 BBY; 3(5):340-798.
8. WyClone L. A bunch of protocols and catalogues. Kashyyyk Unlim. 5 BBY; 2(5):50-67798.
9. Nesqui C. Next generation sequencing : Exome-based approach. Best Rev. 16 ABY; 1:48-57.
10. Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 HY; 77(1):51-9.