UPREGULATED MEMBRANE EXPRESSION OF A CONSERVED VOLTAGE-GATED SODIUM CHANNEL, Nav1.4a AND ELECTRICAL ORGAN DISCHARGE IN ELECTRIC MOUSE, P. pikachu

– – –
PLOS BIOLOGY (March 2013). Vol 11 Issue 3. e1001501 p1-6 pdf download
ABSTRACT
Electrocytes contain membrane proteins that allow the polarization of the plasma membrane, thereby allowing the generation of electricity in animals. It has been long established how electricity is generated in the electric eel, but recent studies found similar electrocytes to be active in electric mice. We aimed to study the basis behind electric discharge in a land animal. We found that the voltage-gated sodium channel, Nav1.4a, was expressed in electric organs of the electric mouse, Pokemon pikachu and the electric eel, Electrophorus electricus. However, Nav1.4a was not expressed in the muscle cells of E. electricus while it was expressed in the muscle cells of P. pikachu and other rodents. We also found that P. pikachu and E. electricus shared similar amino acid substitutions at the nonconserved region of this protein. Voltage-clamp technique gave insight on the much greater potential differences generated by P. pikachu compared to electric eel and finally, microscopy analysis revealed greater Nav1.4a numbers in P. pikachu, potentially correlating with aforementioned greater electric potential generation, which perhaps lead to its capability to discharge electricity readily through air.
AUTHOR SUMMARY
Many species of fish are able to generate weak or strong electric discharges, either for communication or for stunning predator or prey. The electric organ, made of electrocytes, is responsible for generating electric discharge. Electrocytes are thought to be derived from neuronal and muscle cells. The voltage-gated sodium channel, Nav1.4a, is found to be absent in the muscle cells, but is highly expressed in the electric organs of electric fishes. In our study, we looked at Nav1.4a in a species of mouse, P. pikachu, that also generates electricity, but through air instead of water. We compared this electric mouse with electric eel as well as nonelectric rodents. Here, we found that Nav1.4a is expressed in both the muscles and electric organs of P. pikachu. In terms of the amino acid sequence, the channel protein of P. pikachu was more similar to the electric eel rather than the rodents. We then observed that P. pikachu possessed much greater numbers of Nav1.4a and generated a much higher potential compared to the electric eel, which may explain its ability to discharge electricity through air.
INTRODUCTION
The nervous and muscular systems developed shortly after the appearance of the first multicellular organisms, approximately 650 million years ago [1] . In animals with a nervous system, the neurons generate action potentials (AP) and form circuits: furthermore, they possess the capability of releasing neurotransmitters, innervating muscles and directing behaviour [2]. The foundation of electrical excitability in animals is built upon the voltage-gated ion channel. The simplest forms, which include potassium leak and voltage-dependent (K+) channels, appeared 3 billion years ago in bacteria and exist in all organisms today [3]. Voltage-gated ion channels provide a resting potential and repolarize membranes that have been excited. These simple ion channels have, however, evolved further from the six transmembrane (6TM) helices K+ channels into 4x6TM channels, obtaining Ca2+ permeability along the way. From there, Voltage-gated Na+- permeable channels then evolved. Nav channels are the basis for electrical excitability in animals [2].
The polarization of membranes is indeed key to animals that generate electricity. Cells called electrocytes have evolved key muscle membrane proteins that polarize the plasma membrane. The electric tissue in these animals contain membrane proteins that are homologous to those found in other excitable tissues, and especially in the electric eel where electric tissues are invaluable for studying membrane excitability. The electric eel, Electrophorus electricus, is capable of generating electrical potential of up to 600 volts by means of its three well-defined electric organs. The main organ generates high voltage discharges and runs from the viscera of the eel down to the tail, while the adjacent organs emit low electric charges and probably serve in facilitating the eel’s sense of electrolocation [4].
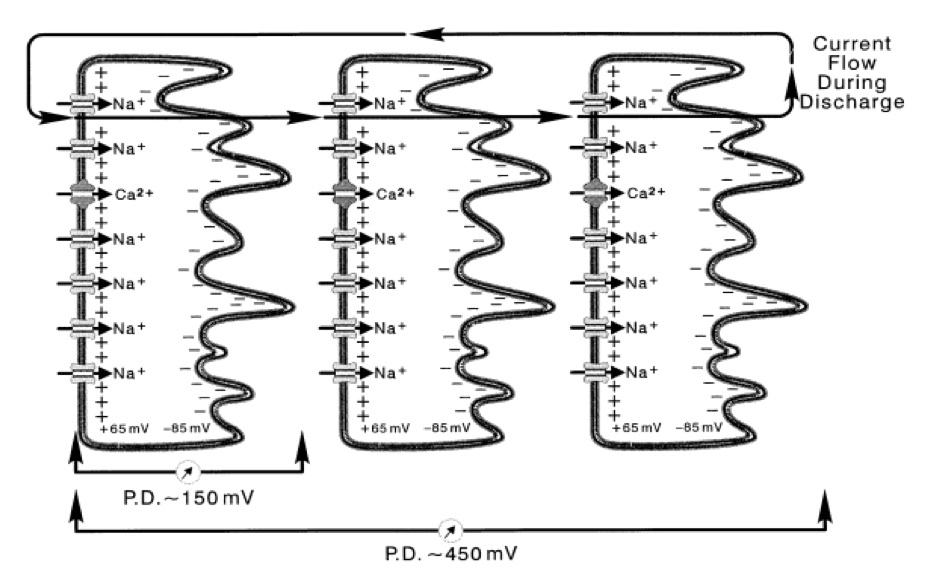
Columns of cells run the length of the main organ and are separated by electrically insulating septa. Electrocytes themselves are multinucleated syncytia, which are stacked one after another to make up those aforementioned columns, thereby allowing the production of pronounced fluctuation in membrane potential. Figure 1 displays an example of how electrocytes are stacked and cumulatively produce a higher potential. We assume a similar set up for electric mice, except that the current circuit is closed by flowing through the air. The lower conductance of air in electric mice led us to study the key difference that allows Pikachu to do so.
Figure 1. [Click Image to Enlarge] Physiological basis of electric organ discharge (EOD). Electrocytes are stacked together in series. The simultaneous stimulation of the electrocytes allow the transcellular potentials to summate. One electrocyte in E. electricus produces a intracellular potential of 150 mV. By stacking in series, potentials summate over the electrocytes. In this figure, three electrocytes of E. electricus build up to a cumulative transcellular potential of 450 mV. For electric fish, the current flows in posterior to anterior direction, and the circuit is closed by flowing out of the water, back to the tail. Derived from Bennet et al, with permission [4].
Over the past few decades it has been reported that certain mouse strains are also capable of generating electricity in a similar fashion to the E. electricus [5]. The most prominent of which is the species of mouse Pokemon pikachu, which has already been the subject of several other studies. Ash Ketchum et al [6]. have shown the existence of electric organs in P. pikachu’s cheeks and tails, where stacks of electrocytes have been found. This has prompted our research group to further investigate P. pikachu’s electrocytes to look for a common ancestral line from which mouse and eels separate. More specifically, we are interested in the Nav1.4 gene that is conserved in the electric mouse and thus, we compare sequences to possibly find homologues between mouse and eel Nav1.4 gene and how the difference contribute to the gene’s function (especially regarding the fact that electric mouse is able to generate electricity on land). Further, we investigate how the Pikachu electrocytes generate the more massive amounts of electricity in comparison to electrophorus electricus and we look at the voltage production by means of voltage-clamp technique. Finally, we are interested in comparing the cell morphology, using confocal microscopy imaging, to provide an answer whether cell size plays a role in electric mice generating higher voltages.
RESULTS
Nav1.4a is expressed in the muscles and EO of P. pikachu
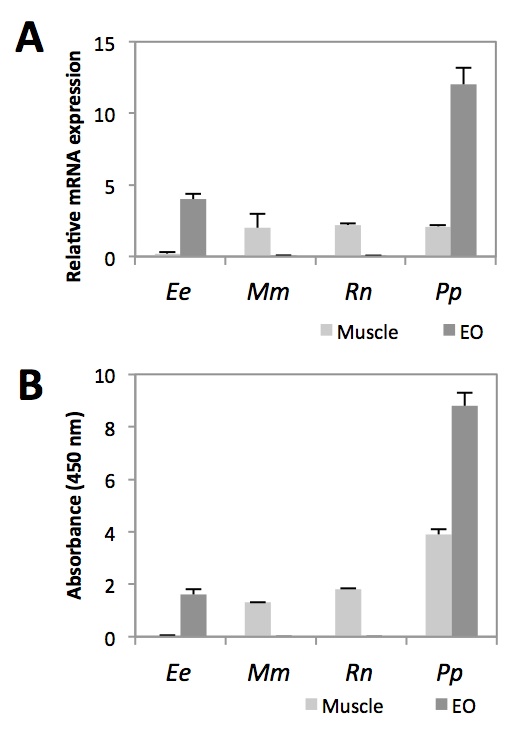
To assess whether Nav1.4a is also expressed in the EO of P. Pikachu, we first measured its transcript levels of Nav1.4a in EO and muscles, and compared that with the transcript levels in E. electricus (Fig. 2A). As we predicted, like E. electricus, Nav1.4a was highly expressed in the EO of P. Pikachu. Studies using ELISA are in agreement with this finding (Fig. 2B). Previous studies have shown that Nav1.4a expression is lost in the muscles of many strains of electric fish, presumably in exchange for its expression in the their dedicated EO [7]. This is consistent with the low levels of Nav1.4 we observed at the transcript and protein levels of muscle tissues in E. electricus. Interestingly, while highly expressed in the EO, Nav1.4a of P. Pikachu was expressed at a level comparable to that of two other rodents, Rattus novigicus and Mus musculus (Fig. 2A). This suggests that phenotypically, Nav1.4a of P. pikachu resembles both electric fishes and rodents.
Figure 2. [Click Image to Enlarge] Comparison of Nav1.4a expression in muscle and EO across four species. A. Relative Nav1.4a mRNA abundance measured by qRT-PCR. Each sample was normalized to beta-action. B. Relative abundance of Nav1.4a protein measured by ELISA. N=5. Ee, Electrophorus electricus; Mm, Mus musculus; Rn, Rattus norvegicus; Pp, Pokemon pikachu.
Nonconserved amino acid sequences in the Nav1.4a of P. Pikachu are similar to the E. electricus variant
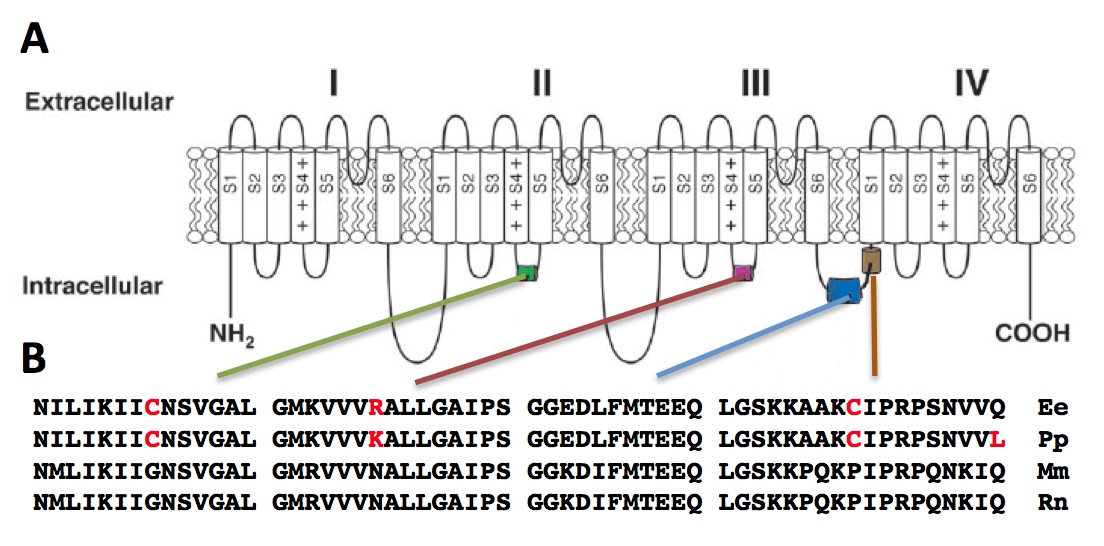
Next, we compared the amino acid sequences of the Nav1.4a of the four species. Overall, the majority of the regions contained conserved amino acid sequences for the four species (data not shown). This is in agreement with previous studies, which have shown that a large portion of the protein contain conserved sequences [7]. It has been suggested that evolution acts to maintain this conservation, as amino acid substitutions in many regions critical for fundamental properties of Na channels lead to often fatal neuromuscular diseases in human [8-9]. We zoomed in our analysis on four intracellular loops located in Domain II, Domain III, and between Domain III and IV (Fig. 3A). These loops have been shown to play roles in sodium channel inactivation, a vital step in the cell electrophysiology and also a determinant step of rate of inactivation [10].
The Glycine to Cysteine substitution in the S4-S5 loop in Domain II creates an additional disulfide interaction, which may affect the conformational changes of the sodium channel (Fig. 3B). For the S4-S5 loop in Domain III, an Asparagine residue is highly conserved for mammals, which forms part of the receptor for inactivation. In both E. electricus and P. pikachu, this residue is replaced with positively charged Arginine and Lysine. There is no evidence showing phenotypic consequences for differences between an Arginine or a Lysine residue Substitution for the highly conserved Proline with Cysteine in the interdomain linker has been shown to perturb channel inactivation in rats [8]. Overall, the amino acid sequence at the intracellular loops for P. pikachu resembled more of E. electricus than the rodents, which are the perceived closer relatives to pikachu.
Figure 3. [Click Image to Enlarge] Comparison of interspecies amino acid sequence of Nav1.4a. A. Schematic drawing of the domains of Nav1.4a. B. Four variable regions of the peptide (S4-S5 linker of Domain II, S4-S5 linker of Domain III, two interdomain linker of Domains III-IV) were compared in greater detail. Ee, Electrophorus electricus; Mm, Mus musculus; Rn, Rattus norvegicus; Pp, Pokemon pikachu.
Individual electrocytes from P. pikachu generates greater transcellular potential compared to E. electricus
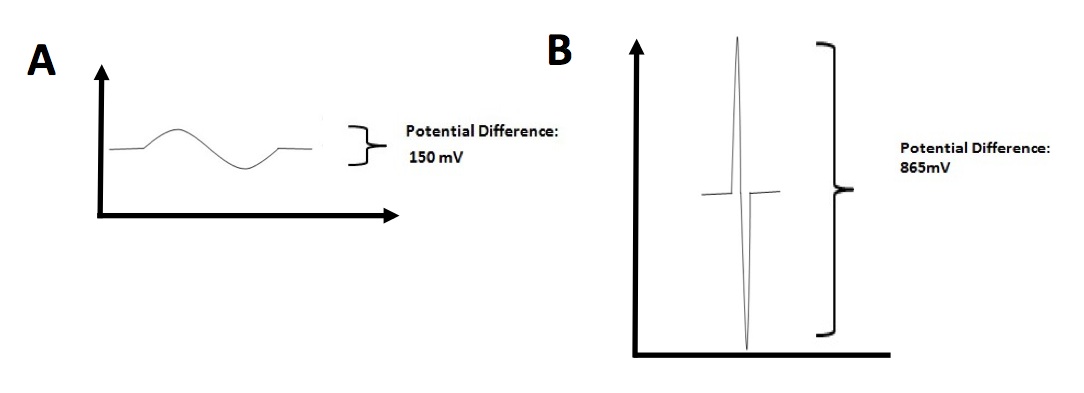
Since Nav1.4a proteins between E. electricus and P. pikachu are similar in terms of amino acid sequence, we next sought to study the physiological property of individual electrocyte isolated from E. electricus (Fig. 4A) and P. pikachu (Fig. 4B). Potential Difference in individual electrocytes were analyzed. Two pieces of information were generated from Fig. 4. The first information was that Potential Difference generated from P. pikachu electrocyte was on average 865 mV which was 4.7 times higher than what E. electricus electrocytes generated (150 mV). The second information is that there was a 10-fold difference in electric discharge duration between P. pikachu (0.01 s) and E. electricus (0.1 s). This data indicates that pikachu’s electrocytes generate higher electric potential difference in a higher frequency than E. electricus.
Figure 4. [Click Image to Enlarge] Time-dependent electric potential difference in individual electrocytes. A. Measurement of potential difference across electrocytes in E. electricus. B. Measurement of potential difference across electrocytes in P. pikachu. Representative measurements from one electrocyte from each species are shown. Other data not shown. Measurements were repeated for 20 electrocytes per species.
Nav1.4a is highly expressed on the membranes of the electrocytes of P. pikachu compared to E. electricus
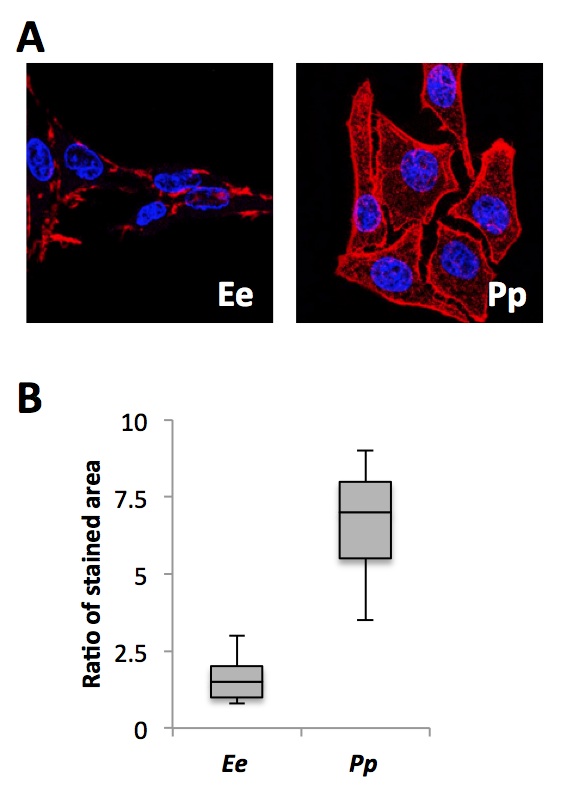
We next sought to determine the cause for the greater transcellular potential and frequency of EOD of P. pikachu compared to E. electricus. EO from both species were isolated and immunostained for Nav1.4a. Using confocal microscopy, the image sections containing ion channel-rich side of the membrane were analyzed. Fig. 5A shows the representative confocal microscopy image of electrocytes from E. electricus and Fig. 5B for P. pikachu after immunostaining. The stained area were normalized with the nuclei counts for quantification. Electrocytes from P. pikachu had significantly greater amounts of stained area per nuclei, indicating that at the single cell level, the electrocytes of P. pikachu contained more membrane-bound Nav1.4a than E. electricus.
Figure 5. [Click Image to Enlarge] Immunostaining of Nav1.4a in E. electricus and P. pikachu. A. Representative confocal microscope images of the two species at 20X magnification. Blue: nucleus; Red: Nav1.4a. B. Image-based quantification. Following color segmentation, the area of red was normalized to the number of nuclei present. For the box plot, the lower whisker, lower box, upper box, and upper whisker each represents the 1st to 4th quartile of the obtained data set. N=20
DISCUSSION
While the existence of mice, with EO, possessing the capabilities of generating electric discharges had been identified decades earlier, the mechanism behind electric discharge through air was poorly understood. We began our study focusing on a voltage-gated sodium channel protein, Nav1.4a, which was expressed in the EO of all known marine electric organisms. We found that Nav1.4a was also expressed in the EO of P. pikachu, located at its cheeks and tail. Interestingly, while electric fishes have lost the Nav1.4a expression in the muscle cells, P. pikachu maintains its expression. Therefore from the standpoint of tissue-specific expression, P. pikachu shares both the characteristics of an electric fish as well as its closely-related rodents. In the case of electric fish, by losing ion channel expression in muscle they have sacrificed some functions of their muscle in return for the greater benefit of electrocommunication and attacking predators or preys through electric discharge. P. pikachu resides in areas where there is high competition for physical fitness, required for constantly battling other species. Therefore, it is not illogical that P. pikachu has been selected to possess both phenotypes – maintaining the motility of a rodent with gained ability to generate electricity.
The analysis of amino acid substitutions confirmed that the Nav1.4a channels in the EO of P. pikachu are more similar to other electric fish, and less similar to the rodent families. The amino acid substitutions shared by P. pikachu and E. electricus are mostly at regions associated with channel inactivation. This perhaps shapes the difference between the ion channels functioning as a mode for relaying information in the body versus a mode for generating electricity.
The sequence similarity between the Nav1.4a in P. pikachu and E. electricus, however, would not explain how one is able to induce electric discharge over air while the other is limited to water. Therefore, functional studies on individual electrocytes were performed to measure the differences in the potential generated by EO in P. pikachu and E. electricus. We have observed that the electrocytes for P. pikachu generated much greater trancellular potential at a higher frequency. Immunostaining was then performed to confirm that P. pikachu electrocytes have far greater abundance of Nav1.4a. It is possible that by having more sodium channels, a greater amount of sodium ions enter the electrocytes once signaled, leading to a faster ion influx, resulting in higher frequency, as well as greater ion influx, and resulting in higher potential. The possible consequence of having electrocytes each generating a transcellular potential of nearly 1 V is that, summed together, the entire EO of P. pikachu may generate up to thousands of voltages of electricity. It may be this high magnitude of voltage that allows this strain of electric mouse to discharge electricity through air.
Future work would revolve around confirming some of the hypotheses stated above. We will first need to determine whether there is a direct correlation between the number of Nav1.4a and the transcellular potential. One way to test this is to treat electrocytes with small molecule inhibitors of sodium channels, at various dosages, and measure whether there are changes in potential associated with the treatment. Secondly we will need to study the physiology of the EO of P. pikachu as a whole. It is possible that the surrounding matrix, or supportive tissues in P. pikachu are allowing for the generation of greater potential. Nevertheless, our work has created a potential link between the molecular biology of marine electric fish and an electric mouse. This may open new fields of research on electric mouse using methods previously developed for electric fish.
MATERIALS AND METHODS
Nucleic acid extraction
Electric organs from the electric eel (Electrophorus electricus) and Pikachu (Pokemon pikachu) were extracted. Neighboring muscle tissues from brown rat (Rattus norvegicus), house mouse (Mus musculus), and Pikachu were extracted. Total RNA were isolated from muscles and electric organs in all species using the miRNeasy mini kit (Qiagen).
cDNA synthesis and qRT-PCR
Random hexamers were used to reverse transcribe the extracted total RNA into cDNA using the SuperScript III first-strand cDNA synthesis kit (Life Technologies). Real-time PCR was performed with Nav1.4a-specific primer/probe set (Universal Probe Library #14, Roche), using the Taqman chemistry (Life Technologies). Beta-actin-specific primer / probe set (Universal Probe Library #64, Roche) was used as endogenous control.
ELISA Assays
Muscle tissues and electric organs were collected from electric eel, mouse, rat, and Pikachu. Levels of Nav1.4a protein in these tissues and organs were determined using ELISA assay kits from SABioscience according to the manufacturer’s instructions.
RNA-Seq and in amino acid sequence
The cDNA obtained from the previous step were also used for RNA-seq. Barcoded fragmented cDNA libraries were prepared using the TruSeq RNA Sample Prep Kit (Illumina). The libraries were then pooled and sequenced on a MiSeq sequencer with MiSeq Reagent Kit v2 – 300 cycles Kit (Illumina). The obtained sequences are then demultiplexed and joined to recreate the full cDNA sequences of Nav1.4a. In silico translation was performed from the cDNA sequences, using ExPASy translate tool, to obtain the amino acid sequences for each species.
Measurement of transcellular potentials
Electrocytes were isolated and were mounted in a Lucite holder in which the cell separates two pools of a modified Ringer’s solution (containing 165 mM NaCl, 2.3 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 1.2 mM K2HPO4, 0.3 mM KH2PO4, 10 mM glucose, pH=7.1). The electrocytes are placed on the holder horizontally, innervated side down, and part of the innervated membrane, exposed through a window (3 X 0.7.5 mm), is in contact with a stream of fresh solution. The rate of flow was such that the solution bathing the innervated side could be changed in a few seconds. Potential differences across either membrane were measured between an intracellular glass microelectrode filled with 3 mM KCl and agar bridges in the outside solutions. Electric potential was calculated through equations established previously [11].
Immunostaining and Confocal Microscopy
Electrocytes from electricus and pikachu were mounted onto glass sides and fixed with 0.4% paraformaldehyde. 10% BSA-PBS was used to block the specimen for 20 min. Goat anti-Nav1.4a antibody (Novarus) was applied at 1:100 at room temperature for 1 hour. After 3 x 10 min washes with PBS, Texas Red-conjugated rabbit anti-goat antibody was applied at 1:1000 at room temperature for 1 hour. DRAQ5 nuclear stain was applied at 1:10,000 for 10 min prior to mounting the coverslip. Stained slides were imaged using a confocal microscope (Nikon). Z-stacks corresponding to different vertical sections of the specimen were imaged. The Otsu’s image-based segmentation method was used to quantify stained nuclei and Nav1.4a area.
REFERENCES
1. Love GD et al (2009) Fossile steroids record the appearance of Demospongiae during the Cryogenian period. Nature 457:718-721.
2. Zakon HH (2012) Adaptive evolution of voltage-gated sodium channels: the first 800 million years. Proc Natl Acad Sci U S A 109 Suppl 1:10619-10625.
3. Anderson PAV, Greenberg RM (2001) Phylogeny of ion channels: clues to structure and function. Comp Biochem Physiol B Biochem Mol Biol 129:17-28.
4. Bennet MVL (1971) Electric organs in Fish. Physiology 5:347-491.
5. Haruka M et al (2001) Generation of electricity in mouse strain Pokemon pikachu. Natural 8921:123-145.
6. Ketchum A et al (2000) Identification of electric mouse. Natural 8890: 133-139.
7. Zakon HH et al (2006) Sodium channel genes and the evolution of diversity in communication signals of electric fishes: convergent molecular evolution. Proc Natl Acad Sci U S A 103:3675-3680.
8. Tian XL et al (2004) Mechanisms by which SCN5A mutation N1325S causes cardiac arrhythmias and sudden death in vivo. Cardiovasc Res 61:256-267.
9. Kellenberger et al (2997) Molecular analysis of potential hinge residues in the inactivation gate of brain type IIA Na+ channels. J Gen Physiol 109:607-617.
10. Numann R et al (1991) Functional modulation of brain sodium channels by protein kinase C phosphorylation. Science 254:115-118.
11. Karlin A (1967) Permeability and internal concentration of ions during depolarization of the electroplax. Proc Natl Acad Sci U S A 58:1162-1167.
Acknowledgements
We thank Francisco Bargala, John Avise, and Georg Strieter for providing previous tissue materials from Pikachu. Thank you also to Francisco Bargala for facilitating the dinner conversation with excellent wines from his vineyards.
Author Contributions
The author(s) have made the following declarations about their contributions: Conceived and designed the experiments: MA CS NL. Performed the experiments MA NL. Analyzed the data: CS NL. Wrote the paper: MA CS NL.
In case it wasn’t clear already, this article is not real.