APOPTOSIS
(August 2004)
Introduction
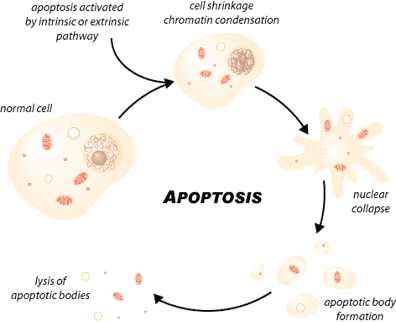
Apoptosis, or programmed cell death, is a highly regulated process that allows a cell to self-degrade in order for the body to eliminate unwanted or dysfunctional cells. During apoptosis, the genome of the cell will fracture, the cell will shrink and part of the cell will disintegrate into smaller apoptotic bodies. Unlike necrosis, where the cell dies by swelling and bursting its content in the area, which causes an inflammatory response, apoptosis is a very clean and controlled process where the content of the cell is kept strictly within the cell membrane as it is degraded [1]. The apoptotic cell will be phagocytosed by macrophages before the cell’s contents have a chance to leak into the neighbourhood [1]. Therefore, apoptosis can prevent unnecessary inflammatory response.
Apoptosis is essential to embryonic development and the maintenance of homeostasis in multicellular organisms. In humans, for example, the rate of cell growth and cell death is balanced to maintain the weight of the body. During fetal development, cell death helps sculpt body shape, separating digits and making the right neuronal connections. In the immune system, cell death eliminates B cells and T cells that elicit autoimmune response and selects the most efficient lymphocytes to encounter an antigen in the process of affinity maturation.
Pathways
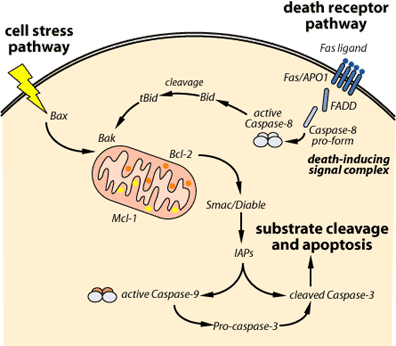
Apoptosis can be triggered in a cell through either the extrinsic pathway or the intrinsic pathway. The extrinsic pathway is initiated through the stimulation of the transmembrane death receptors, such as the Fas receptors, located on the cell membrane. In contrast, the intrinsic pathway is initiated through the release of signal factors by mitochondria within the cell.
The Extrinsic Pathway: In the extrinsic pathway, signal molecules known as ligands, which are released by other cells, bind to transmembrane death receptors on the target cell to induce apoptosis. For example, the immune system’s natural killer cells possess the Fas ligand (FasL) on their surface [2]. The binding of the FasL to Fas receptors (a death receptor) on the target cell will trigger multiple receptors to aggregate together on the surface of the target cell. The aggregation of these receptors recruits an adaptor protein known as Fas-associated death domain protein (FADD) on the cytoplasmic side of the receptors. FADD, in turn, recruits caspase-8, an initiator protein, to form the death-inducing signal complex (DISC). Through the recruitment of caspase-8 to DISC, caspase-8 will be activated and it is now able to directly activate caspase-3, an effector protein, to initiate degradation of the cell. Active caspase-8 can also cleave BID protein to tBID, which acts as a signal on the membrane of mitochondria to facilitate the release of cytochrome c in the intrinsic pathway [3].
The Intrinsic Pathway: The intrinsic pathway is triggered by cellular stress, specifically mitochondrial stress caused by factors such as DNA damage and heat shock [3]. Upon receiving the stress signal, the proapoptotic proteins in the cytoplasm, BAX and BID, bind to the outer membrane of the mitochondria to signal the release of the internal content. However, the signal of BAX and BID is not enough to trigger a full release. BAK, another proapoptotic protein that resides within the mitochondria, is also needed to fully promote the release of cytochrome c and the intramembrane content from the mitochondria [4]. Following the release, cytochrome c forms a complex in the cytoplasm with adenosine triphosphate (ATP), an energy molecule, and Apaf-1, an enzyme. Following its formation, the complex will activate caspase-9, an initiator protein. In return, the activated caspase-9 works together with the complex of cytochrome c, ATP and Apaf-1 to form an apoptosome, which in turn activates caspase-3, the effector protein that initiates degradation. Besides the release of cytochrome c from the intramembrane space, the intramembrane content released also contains apoptosis inducing factor (AIF) to facilitate DNA fragmentation, and Smac/Diablo proteins to inhibit the inhibitor of apoptosis (IAP) [4].
Cancer
Cancer may arise from the dysfunction in the apoptotic pathway. Due to the sensitivity of the intrinsic pathway, tumors arise more often through the intrinsic pathway than the extrinsic pathway [5]. In the intrinsic pathway, a very common cause of tumorgenesis is mutation of the p53 protein [5]. Besides regulating apoptosis, p53 also regulates the check points in the cell cycle, DNA repair, senescence and genomic integrity [6]. Any mutation that causes p53 to lose any of its function will induce tumorgenesis by letting the cell grow indefinitely without any regulation. Another important factor in tumorgenesis is the balance between the proapoptotic and antiapoptotic members of Bcl-2 family. In a tumor cell, a mutation of Bcl-2 gene that results in increased expression will suppress the normal function of the proapoptotic proteins, BAX and BAK [5]. On the other hand, if a mutation on the BAX or BAK genes cause a downregulation of expression then the cell will also lose its ability to regulate apoptosis, again causing tumorgenesis [5].
HIV
Human immunodeficiency virus (HIV) contains many gene products that can kill the infected immune cells by activating the apoptotic pathway so that it might weaken the host’s immunity. Mainly, HIV can induce apoptosis through cell fusion (syncytia) or through its gene-encoded products. In preparation for syncytia, HIV will express one of its encoded proteins, Env, on the surface of the infected host cell. Env will bind to CD4 receptors on an uninfected cell and trigger cell fusion [7]. The fused cell will upregulate one of its cell-cycle regulating proteins, cyclin B-CDK1, which leads to the disruption of the cell cycle [7]. The disrupted cell cycle leads to more p53 production and triggers apoptosis through the intrinsic pathway by the upregulation of BAX. Besides syncytia, HIV can induce apoptosis directly through the proteins that its genes encode. For instance, Vpr can induce the intrinsic apoptotic pathway by disrupting the mitochondrial membrane potential and promoting the release of cytochrome C. Tat, on the other hand, promotes apoptosis by downregulating BCL-2 and upregulating caspase [8 7].
Conclusion
The apoptotic pathway is one of the most sophisticated pathways discovered in the cell to date. Its activity is tightly regulated and monitored by the cell. Recent advances and understanding of the apoptotic pathway have led to better and more innovative treatments against cancer and other diseases. However, the detailed mechanism of the apoptotic pathway is still waiting to be elucidated.
References
1. Raff, Martin. Cell suicide for beginners. Nature 396(1998): 119-122.
2. Csipo, I., Montel, A. H., Hobbs, J. A., Morse, P. A., and Brahmi, Z. Effect of Fas+ and Fas- target cells on the ability of NK cells to repeatedly fragment DNA and trigger lysis via the Fas lytic pathway. Apoptosis 3(1998): 105-114.
3. Adrain, C., Creagh, E. M., and Martin, S. J. Caspase Cascades in Apoptosis. Caspases-their role in cell death and cell survival. Ed. Marek Los and Henning Walczak. Moleculare Biology Intelligence Unit 24. New York: New York, 2002. 41-51.
4. Hague, A., and Paraskeva, C. Apoptosis and disease: a matter of cell fate. Nature Cell Death and Differentiation 3 September 2004: 1-7.
5. Johnstone, R. W., Ruefli, A. A., and Lowe, S. W. Apoptosis: a link between cancer genetics and chemotherapy. Cell 108(2002): 153-164.
6. Schmitt, C. A., Fridman, J. S., Yang, M., Baranow, E., Hoffman, R. M., and Lowe, S. W. Dissecting p53 turmor suppressor functios in vivo. Cancer Cell 1(2002): 289-91.
7. Gougeon, Marie-Lise. Apoptosis as an HIV strategy to escape immune attack. Nature 3(2003): 392-404.
(Art by Jen Philpott)